We will now explore in more depth the laws that rule the behaviour of the
atomic and sub-atomic world, the laws of Quantum Mechanics
We have already encountered one of the first properties (which in fact is the
justification for the term quantum) : when dealing with the elementary
constituent of matter and energy, one should abandon the concept of
continuous variables, which was one of the pillars of Classical (as opposed to
Quantum) Physics, but should instead think in terms of discrete (i.e
quantized) entities.
As we proceed, we will become aware of two more unexpected (and puzzling)
properties of the quantum world:
- in the sub-atomic world, it will not be possible anymore to make exact
numerical predictions concerning the evolution of physical phenomena, but it
will only be possible to estimate the probability of different outcomes
- we will also learn that all sub-atomic objects have a weird "double
personality", since thay can exhibit at the same time both wave and particle
properties.
Determinism vs. Probabilism : in Classical Physics (think e.g. of Newton's law
of gravity), it was a fact that, given the knowledge of an object's position
and velocity and of the forces to which the object was subjected to, it
was possible, at least in principle, to predict the object's position and
velocity at any future instant. In this way, for instance, astronomers could
predict that a given comet would return after so many decades, or that a solar
eclipse was due on a given date, etc.
This deterministic , or mechanical, view of the universe originated
some interesting philosphical discussion about the meaning of free will:
it could be argued that the evolution of the universe, therefore of every object
within it, was completely determined by its conditions at a given instant,
therefore there was no room for free will....
Rather than pursuing this interesting discussion, we will examine what happens
in the quantum world, but before doing so, we must get a better understanding
of what we mean by knowing an object's position and velocity.
It is a fact that, to determine the position of an object, we must somehow
"see" it. Note that we do not necessarily need to use visible light to
"see":
a police radar will send a train of radar waves against your car to measure
its velocity
oceanographers will map the sea bottom by bouncing sound waves off it,
etc.
A study of these and other examples would convince you that any type of
measurement implies the transfer of some amount of energy to the object being
measured. Most often, this energy is carried by some type of wave.
One could nevertheless estimate that, in the macroscopic world, this amount
of energy transferred is completely negligible.
To aid in understanding the next steps, we will state without proof a
General Principle of Optics (and any other wave-mediated measurement):
a wave of a given wavelength cannot locate an object with precision superior
to the actual value of wavelength (therefore, in order to make more and more
accurate measurements, one would need waves of shorter and shorter wavelength,
i.e. higher and higher frequencies).
Given all this, let us see what happens if we try to determine the position of
an electron : in order to "see" it, we would have to hit it with a quantum of
light (a photon) and having the photon bouncing back to our eye (or our
measuring instrument). But unavoidably, in the electron-photon "collision",
the photon would transfer some of its energy to the electron, therefore
modifying its velocity : in the sub-atomic world therefore
the process of measuring an object's position unavoidably affects the
state of the object.
The situation gets worse and worse if we try to measure the object's position
with higher and higher precision : from the general principle presented above,
we will need to "illuminate" the electron with higher and higher frequency
photons, therefore (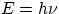 ) with photons which are more and more energetic,
and in doing so we will affect more and more the original electron's
velocity.
) with photons which are more and more energetic,
and in doing so we will affect more and more the original electron's
velocity.
These types of considerations were solidified into one of the basic rules of
Quantum Mechanics, Heisenberg Uncertainty Principle, stating
that:
it is impossible to measure simultaneously position and velocity of a particle
with arbitrarily good precision. The better knowledge we can gain of one
variable the less the other variable will be known. In formula:
with 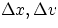 = precision in knowledge of the particle's position
and velocity, h = Planck's constant, m = mass of the particle.
= precision in knowledge of the particle's position
and velocity, h = Planck's constant, m = mass of the particle.
(mathematical parenthesis to examine a function of the type xy=const)
Why do we have to worry about Heisenberg's principle only in the sub-atomic
world?
Example 1 : with what uncertainty can we determine the velocity of a track
racer when he crosses the finish line ?
Example 2 : with what uncertainty can we determine the velocity of an electron
inside an atom?
One immediate consequence of Heisenberg's principle is that we have to abandon
the concept of determinism : given that there is an intrinsic limit in the
accuracy of our measurements, then we cannot make exact predictions about the
outcome of physical events. The only thing we can do is to estimate the
probability of various possible outcomes. This concept of probablity
is one of the foundations of the whole theory of Quantum Mechanics, and has
generated (and still does) endless debates over the real nature of the
universe. The most famous exchange:
Einstein : I refuse to believe that God plays dice with the universe
Bohr's reply : Albert, stop telling God what to do...
It is worth pointing out that Heisenberg's Principle appears to have a very
fundamental nature, and is not something intrinsic to our limitations
in measuring positions and velocity. A corroborating fact :
it could be shown that an alternate way of writing Heisenberg Principle is
E=Energy, t=time, the uncertainty in the Energy of an object is related to the
uncertainty in the time when the object had that energy. Elementary particles
seem to be aware of this fact since we know that, in the sub-atomic
domain, energy conservation can be violated, provided this is done over a short
enough time, consistently with the Uncertainty Principle.
Particle or Wave ?
Let us begin with light. We have repeatedly stated that light (as well as all
the rest of the ElectroMagnetic radiation) is a wave. How do we know that?
Apart from the obvious cases when the oscillations can be directly observed
(e.g. sea waves), waves have an unmistakeable property that unmasks them:
waves interfere. The standard experiment consists of sending the
alleged wave through two gaps, so that two waves are formed out of the
original one, and observe whether interference (constructive and destructive)
does take place.
Interference effects will be best observed when the size of the gaps and their
separation are of the same order of magnitude as the wavelength of the wave
being examined.
Measurements of interference phenomena, among others, allow to perform a direct
determination of the wavelength of the various colours of light.
Similar experiments can be performed with any other portion of the
ElectroMagnetic spectrum, provided the correct size of slit is chosen.
Example: X-rays
Remember the early experiments showing the presence of electrons? In those
early days it was discovered that, when the "cathode rays" (i.e. high speed
electrons) hit a metal target, a new form of radiation is generated. This
radiation is not affected by electromagnetic field, therefore it is electrically
neutral, and it is very penetrating. Not knowing its nature, it was originally
named X-ray. As time progressed, there was more and more suspicion that X-ray
were a higher energy section of the electro-magnetic spectrum. Their energy
could be estimated from the energy of the electrons used to generate them: to
generate X-rays, electrons were accelerated through voltages of a few thousand
Volts, therefore the typical energies should have been of the order of keV.
This is about a thousand times more energetic than light waves therefore
(remember, 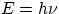 ) their frequency had to be 1000 times higher,
i.e. their wavelength 1000 times shorter, somewhere around 10-10
m.
) their frequency had to be 1000 times higher,
i.e. their wavelength 1000 times shorter, somewhere around 10-10
m.
In order to verify their wave nature, it would have been necessary to send them
through some slit 10-10 m wide !! Such a grid cannot be made by man, but
it can be found in nature. We have seen that typical atom dimensions are
around 10-10 m. The regular spacing of atoms in a crystal did provide
exactly the required tool. X-rays were sent through a crystal, and interference
pattern was observed. This confirmed that X-rays were waves of exactly the
expected wavelength.
We now also understand how X-rays are generated, in fact they are produced in
two distinct processes:
- 1.
- high energy electrons traveling in vacuum undergo sudden deceleration
when hitting the metal target material and, remember, an accelerated charge
radiates....this process generates a continuum spectrum of X-rays frequencies.
- 2.
- high energy electrons can also excite atomic electrons in the target to
a high energy orbit (as described by Bohr). Energies typical of electron
transitions in heavy elements are much larger than those typical of light
elements (Hydrogen, Helium, etc.). For heavy elements, typical spectral lines
have energies in the keV, i.e. in the X-ray, range.
Observation of the X-ray spectra show both a continuum distribution and some
discrete lines.
Everyone would then have been happy to accept that light and other
electromagnetic radiation were waves, until the Photoelectric Effect
came into the scene.