Photoelectric effect : shining light onto a metal plate can cause electrons to
be pulled out of the metal (demonstration : a light beam will start a current in
an otherwise open circuit)
So far, this is not surprising : we know that light is energy, and it is not
inconceivable that some of the electrons in the metal can receive enough energy
to be pulled out of the material. The problem was that the details of the
phenomenon were completely inconsistent with the behaviour of a wave. For
instance, the energy carried by a wave is related to its amplitude (i.e. the
intensity of the light). In the experiments, it was seen that the capability
of initiating the photoelectric effect was not depending upon the light
intensity, but on its colour (blue light would extract electrons, red wouldn't).
As we mentioned in an early lecture, the only explanation for the observed
behaviour was to assume that (Einstein 1905), in this process light was not
behaving as a wave, but as a distinct particle (a quantum of energy).
How can something be at the same time a wave and a particle? this remains one
of the open puzzles of Quantum Mechanics. The recommended approach is just to
accept it as a fact, without trying to visualize it with some picture derived
from our sensory experience.
The attempt to clarify such behaviour has continued, and the puzzle has been
confirmed by the most recent investigations. In a recent experiment, a double
slit was illuminated by light of extremely low intensity, such that it could
be assumed that only one photon at the time was being generated. Even so,
prolonged exposure revealed the characteristic interference pattern.
How can a single particle go through two slits simultaneously? How can a
particle interfere with itself? Maybe one day a better answer will be available,
but for now we have to be satisfied with this puzzling situation.
In spite of these open questions, scientists have been able to exploit the
photoelectric effect in a variety of applications:
- door openers, burglar alarms
- light meters for photographic cameras
- signal transmission (e.g. telephone communications) by means of fiber
optics and photoelectric converters
- X-ray detectors (CAT scan)
- etc.
CAT : Computerized Axial Tomography
Tomography : "picture by means of slices" (same "tomos" greek root as "atom")
Axial : the object to be visualized is "sliced" axially, that is the slices are
perpendicular to an ideal axis, going from head to feet.
Computerized : need computer to cope with complexity of picture reconstruction
and high rate of input data.
CAT is the up to date, high-tech version of X-ray imaging. In the standard
X-ray, the detector that visualizes the image is a photographic film. One only
gets a flat image (a two-dimensional projection), and a fairly massive dosage
of radiation is required. Replacing the photographic film with, very sensitive,
photoelectric detectors, one can afford to illuminate the body "slice by slice"
with suitably directed X-ray pulses of very short duration. Feeding the
X-ray absorption data relative to each slice to a computer, a 3D-image of the
subject can be reconstructed.
OK, so we have learnt that waves are particles. The next surprise will be that
particles are waves
Let us go back to Bohr's model of the atom. In spite of its success, Bohr's
picture did not completely clarify the issue of electrons behaviour within the
atom. To start, Bohr was able to account properly for the spectral lines of the
Hydrogen atom, but his equations were not consistent with the observed spectra
for more complex substances. Moreover, his model did not address the question
of why only certain discrete orbits were allowed, his model only stated that
this is how things are in the atom, without trying to say why.
The impasse was broken when a new, rather unconventional, hypothesis was put
forward by Louis de Broglie : turning around Planck and Einstein idea that
(for electromagnetic waves) a given frequency is associated with a given energy,
de Broglie suggested that for particles, a given (kinetic) energy is
associated with a frequency (i.e. wavelength). Even though we will not write it
down, you should be aware that the relation between energy and frequency is not
the same as the one valid for photons (i.e. 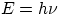 ). Still, the basic
principle is the same:
). Still, the basic
principle is the same:
higher energy = higher frequency.
Again, because of the smallness of Planck's constant, the wave-like nature of
particles only becomes manifest in the sub-atomic world.
How does this affect the behaviour of electrons inside the atom? To get the
answer we must remember two basic facts:
- 1.
- a vibrating body of a given length l (e.g. a guitar string) can only
have stable vibrations at well defined wavelengths, given by :
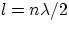 ,that is
,that is 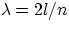 .
. - 2.
- for a body orbiting with constant velocity, a given orbital radius, i.e.
a given length of circumference, corresponds to a well defined energy value.
Combining these two facts together, and assuming that an electron of a given
energy has associated to it a given wavelength, one can reach the following
conclusion:
an electron orbiting around the nucleus cannot have any arbitrary value of
energy (i.e any arbitrary value of orbit length): the only allowed
configurations are the ones for which the length of the orbit corresponds to
integer number of half wavelengths (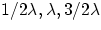 ,
etc.)
,
etc.)
As surprising as it was, the idea of particles exhibiting wave properties
received numerous experimental confirmations. In particular, it was shown that
it does not only apply to electrons bound in atoms but also to free electrons:
sending an electron beam onto a crystal lattice created the same interference
pattern as it had been observed for X-rays.
The conclusion is that all sub-atomic objects share this wave-particle dual
nature in an equal fashion, and either property can be put into evidence by
performing the appropriate experiments. It is interesting nevertheless that,
while electro-magnetic radiation appears more wave-like at lower energies
(e.g. radio waves) and more particle-like at higher energies (e.g. high energy
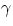 rays), on the contrary, "particles" (e.g. electrons) are more
particle-like at low energies and wave-like at high energies.
rays), on the contrary, "particles" (e.g. electrons) are more
particle-like at low energies and wave-like at high energies.