


Next: About this document ...
Lecture 17
Heat Transmission
Heat Transmission con occur via Convection, Conduction or Radiation. Convection is the dominant
process in fluids (liquids and gases), Conduction acts mostly in solids (but also in liquids and, to some
extent, in gases). Heat transfer by radiation can take place through a vacuum , as well as through any
medium transparent to radiation.
Convection
The mechanism for convection is the density difference in regions of a fluid at different temperatures,
and the consequent action of gravity. Take a fluid at a constant temperature throughout and heat some
region of it. In the warmer region density will decrease and the fluid in that region will rise (according
to Archimedes principle). Simultaneously, cooler fluid will move in to fill in the space vacated by the
warmer fluid. The effect of such convective currents is to transport thermal energy throughout
the fluid.
Typical instances of convection are e.g. water being heated in a pan or the formation of cumulus clouds.
In several applications, one can also have forced convection, e.g. when using a fan or a pump
(to be correct, the radiator in you car should not be called Radiator but "Convector").
Although conceptually easy to understand, it is not easy to describe convection in a quantitative way.
Conduction
While in convection heat is carried by an actual displacement of matter within the fluid, in conduction
only heat is transferred, without any transportation of matter taking place. To interpret the phenomenon
of heat conduction at the atomic level, we should be aware that temperature of a body is related to the
average kinetic energy of its molecules. In the contact region between a colder and a warmer body,
collisions among molecules will have the effect to transfer some of the (thermal) energy from the higher
to the lower temperature. To convince yourself of this you might think of an elastic collision between two
molecules: if they have initial velocities V and v (with V larger than v), momentum and energy
conservation would show that, by effect of the collision, the velocities just get exchanged, so that the
hotter body loses some of its energy and the cooler gains some. When the two bodies reach thermal
equilibrium (i.e. they are at the same temperature) heat exchange still takes place in principle, but, in
the average, as much heat is lost as it is gained, and the body remains at a constant temperature.
But to analyze heat conduction quantitatively it is not necessary to think in terms of collisions among
molecules. Instead, we can easily write the basic expression representing the amount of heat Q transmitted
across a given thickness of material :
Q = k A t
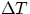
/L
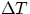 , L is the material
thickness and t is the elapsed time. k is a constant, typical of the material, which represents how well
(or how badly) the material can transmit heat. According to the application, we will want to
employ either very good conductors (e.g. in a frying pan) or good insulators (for the pan's handle).
In may applications, dealing mainly with construction and home insulation, the expression above is
written as
, L is the material
thickness and t is the elapsed time. k is a constant, typical of the material, which represents how well
(or how badly) the material can transmit heat. According to the application, we will want to
employ either very good conductors (e.g. in a frying pan) or good insulators (for the pan's handle).
In may applications, dealing mainly with construction and home insulation, the expression above is
written as
Q = A t
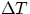
/R
When dealing with conduction across layers of different material, the useful rule to remember is that,
at equilibrium, the same amount of heat has to go through each material.
Radiation
Every object, no matter at how low a temperature (except for absolute zero) emits some
amount of energy in the form of electromagnetic radiation, i.e. electromagnetic
waves. We haven't yet studied neither waves not electromagnetism, but for now let us just accept the
idea that electromagnetic waves can manifest themselves in many different forms, ranging from, in
increasing energy, radio waves, microwaves, infrared, light, ultraviolet, X-rays and
gamma-rays. The member of this family usually associated with heat and temperature is the infrared
radiation, since this type of radiation is felt by our bodies as heat, and the range of
radiation emitted by bodies at ordinary temperatures is peaked mainly in the infrared region (but all
other types of electromagnetic radiation do transfer energy and consequently increase the temperature of
an object, you can get burnt equally well by a laser producing purely visible light or by UV rays).
If a body at a given temperature continuously emits radiation, that is if it emits some of its internal
thermal energy, then, if left to itself, it would gradually cool down (this is in fact what happens
if we have a hot body in a cooler room). But, when in equilibrium with its surroundings, an object
maintains its temperature; this must mean that the body, in addition to radiating away energy, must also
be able to absorb it from its surroundings. And this is exactly what happens: when in thermal equilibrium
with its surroundings a body at the same time absorbs and emits radiation at the rate of
P = Power = Energy/time = Q/t =
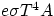
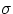 is a universal constant, and e is a number between 0 and 1 that depends upon the properties of the
emitting/absorbing surface.
is a universal constant, and e is a number between 0 and 1 that depends upon the properties of the
emitting/absorbing surface.
It is important to understand the role and meaning of e : let's take a certain object, and "shine" it with
a certain amount of electromagnetic radiation (e.g. light, or infrared, etc.). Experience tells us that
different objects will absorb the radiation in different amounts : we define as emissivity e the
ratio of energy absorbed by the body to the energy received by it. Obviously e will range
between 0 and 1 (and what happens to the energy that is not absorbed? it is reflected).
Now you would be entitled to ask : why did I call this ratio emissivity and not, e.g. absorptivity
? The reason is that there is a one to one correspondance between energy absorbed and energy emitted :
the rate of energy that a body absorbs is identical (for the same temperature) to the rate it will emit,
i.e. the formula above applies equally well to emission and absorption. But do not be confused: the actual
value of emitted energy depends upon the temperature of the emitting body, while that of absorbed energy
depends upon the "temperature" of the energy reaching the body. In formula



Next: About this document ...
Sergio Conetti
11/17/1999