


Next: About this document ...
Lecture 18
Ideal Gas Law
I will assume that you are familiar with the following items :
- atoms and molecules
- elements and compounds
- periodic table of the elements
If you are not familiar with isotopes, here is a quick reminder : atoms consist of a certain number of electrons
orbiting around a central nucleus, which contains protons and neutrons. The chemical properties of any element
depend exclusively on the number of electrons, and the number of electrons is determined by (in fact is equal to)
the number of protons. Consequently, the chemical properties (i.e. what characterizes the individuality of
each element) as well as most physical properties are determined by the number of protons. Two atoms containing
the same number of protons, but different number of neutrons, will have identical chemical properties, therefore
they will be the same element. As they occupy the same place in the periodic table, they are called isotopes
(greek for "the same place"). If we now the position of an element in the periodic table (i.e. the number of
protons in its nucleus), we can determine the number of neutrons: for instance we know that carbon
occupies position 6 in the table, therefore what we call carbon-12 has 6 protons and 6 neutrons, while carbon-13
will have 6 protons and 7 neutrons.
Moles
The book introduces moles in a somewhat unconventional, but rather practical way, and we can follow it : we call
a mole of a given substance an amount of it containing 6.022 x 1023 elementary units (e.g. atoms or
molecules) of that substance. But remember to specify what the elementary units
are. You probably know that e.g. Hydrogen and Oxygen are not found in nature as simple atoms but as bi-atomic
molecules O2 and H2. If I talk about a mole of Hydrogen, I should specify whether I mean a mole of Hydrogen
atoms (6.022 x 1023 atoms) or a mole of Hydrogen molecules (2 x 6.022 x 1023 atoms).
You are entitled to wonder where does 6.022 x 1023 come from, and probably you already
know the answer : historically, a mole was defined as a quantity (in grams) numerically equal to the atomic mass
of the substance, and atomic mass of 1 was (eventually) defined as 1/12 of the mass of Carbon-12. The 6.022 x
1023 (Avogadro's number) then comes from the number of grams in one mole (e.g. 12 for Carbon-12) divided by
the mass (in grams) of a single C-12 atom,
NA = 12/(1.99 x 10-23g) = 6.022 x 1023
Historically, it took a long time before actual values for Avogadro's number and atomic masses (in
grams) could be determined; but Avogadro started it all with his hypothesis that equal volumes of gases
at the same pressure and temperature contained equal numbers of "molecules" (latin for "small mass"). Comparing
the weights of equal volumes of different gases allowed to determine at least relative values of atomic masses.
Ideal Gas Law
Armed with the knowledge of Avogadro hypothesis, we can do some experiments with gases : let us put in a container
a given amount of gas at a certain temperature, and measure its pressure. Then let us add to our container an equal
amount of gas and remeasure the pressure: we will find a value twice as big. We then conclude that pressure is
proportional to the number N of gas molecules (or if you prefer to the number n of moles, since each mole contains
exactly the same number of molecules)
Next: when introducing the absolute zero, we had also seen that when kept at a constant volume, the pressure of a
certain quantity of gas is proportional to its absolute temperature
Finally, it would also be straightforward to see that, when keeping the temperature constant, pressure and volume
change in inverse proportion: if I reduce the volume of a given amount of gas its pressure increases and viceversa
the pressure decreases if the gas is allowed to expand, therefore
Putting it all together, one gets
It would be easy to determine the value of the constant of proportionality, i.e. by introducing a known quantity of gas
(e.g. 1 mole) in a given volume and measuring simultaneously its pressure and temperature. The result is the universal
constant R = 8.31 J/(mol K), and the ideal gas law can then be written as
PV = nRT
or, alternatively,
PV = NkT
with N = total number of molecules in the gas sample and
k = Boltzmann constant = R/NA = 1.38 x 10-23J/K .
Pressure and molecular motion
You were told a few times that what we measure as pressure in a gas is in fact the effect of extremely large amounts of
collisions of gas molecules against any surface. Now we have all the tools to explore this quantitatively : once we know
how many molecules there are in a given gas volume we can estimate the average number of collisions per unit time
and the average force exerted on any surface (the key to the problem is the relation 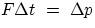 ).
I will not repeat the derivation, but make a few comments to what you find in the book:
).
I will not repeat the derivation, but make a few comments to what you find in the book:
- you are told that, when colliding elastically with the walls of a hypothetical cubic container, the velocity of
a given molecule changes from v to -v. Why is that ? Well, it is certainly in agreement with experience, if you throw
a rubber ball against a wall, it will bounce back (in the perfectly elastic case) with equal and opposite velocity.
But the same result could be easily proved by imposing, as usual, momentum and kinetic energy conservation. In the
limit of an object of mass m hitting an obstacle of infinitely large mass, the solution to the combined equations
would exactly show that the colliding particle acquires a velocity equal and opposite to the incoming one (and,
consequently, the momentum transferred to the wall is
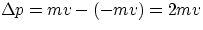 ).
).
- in getting its final result, the book suggests than, in the average, only 1/3 of the particles will hit a given
wall in a given time (being this a 3-dimensional problem). This might be true, but what is not true is that the velocity
of the incoming particle is always perpendicular to the wall (as assumed in the book). A more correct statement would be
that, on average, if v2av is the mean square molecule velocity, v2av/3 is the mean square value of the
velocity component perpendicular to any given direction (i.e. if the average velocity is v, one has
vx=vy=vz=v/3).
Anyway the result one gets is
PV = 2/3 N Ekinav
which relates the pressure and volume of a gas to the total number of molecules and their average kinetic energy. When
combining with the ideal gas law, we get
Ekinav = 3/2 kT
which is quite a remarkable result : just by measuring the temperature of a gas we can determine the average velocity
of its molecules, a quantity that would be extremely difficult to measure !! And, in some special case, we can go even
further : in a gas, molecules carry a certain amount of mechanical energy. In the more general case, this can be in
the form of kinetic translational energy, rotational energy (molecules, having non zero spatial dimensions, can and do
rotate), as well as elastic potential energy (in a molecule, atoms can vibrate around a position of equilibrium in a
harmonic-like fashion), and it would be very difficult to estimate each of these quantities. But in a mono-atomic
gas (e.g. noble gases like Helium, Argon, etc.) vibrational and rotational energies are absent and all of the gas
internal energy is in the form of translational kinetic. We can then say that the total internal energy U of a
mono-atomic gas is given by
U = 3/2 NkT = 3/2 nRT
another rather remarkable result.
Diffusion
Have you ever thought that when you smell a certain substance what is happening is that some molecules of it reach
your nose ? And how can
this happen if the source of the smell is far away? In many cases, convective currents are involved but, even in the
absence of convection, random collisions with, e.g., air molecules contribute to spread molecules of an odoriferous
substance, slowly but surely, uniformly over large distances. This process is called diffusion, and its
quantitative treatment follows that of heat conduction, physicists are cunning, (or is it lazy?), when they have a
successful tool they try to apply it to all sort of different situations.... Fick's law of diffusion states that
the mass m of diffused substance over a path of length L, cross-section A in a time t is given by
m = D A t
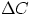
/L
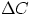 is the difference in concentration between the two ends of the diffusion path.
is the difference in concentration between the two ends of the diffusion path.



Next: About this document ...
Sergio Conetti
11/22/1999