


Next: About this document ...
Physics 201, Midterm Exam 3Nov. 30, 1999
- 1.
- What is the largest value of absolute pressure inside
your mouth that will allow you to sip a drink (density = 1 g/cm3) through
a straw extending vertically 10 cm from the drink surface to your mouth ?
*a) 100300 Pa
b) 1000 Pa
c) -1000 Pa
d) 102300 Pa
*e) 100299 Pa
(note: there was a typo in the test text, answer e was meant to be 102299;
because of this, I allowed both a and e as valid solutions)
- 2.
- You replace the spring of a mechanism with a new one
having a spring constant half the original one. For the same force applied to
the spring
*a) the new spring will stretch twice as much and the work involved will also
double
b) the new spring will stretch half as much, and the work will be half
c) the new spring will stretch twice as much and the work involved will be
4 times bigger
d) the new spring will stretch twice as much but the work involved will be
the same
e) none of the above
- 3.
- Two steel balls of different mass and at different
temperature are brought into contact. When they reach thermal equilibrium,
which one has experienced the larger temperature change ?
a) the hotter one
b) the colder one
*c) the lighter one
d) the heavier one
e) not enough information
- 4.
- The average kinetic energy of individual gas molecules
depends only on the ....... of the gas
a) mass
b) volume
c) density
*d) temperature
e) pressure
- 5.
- After a diver jumps off the platform and before he
hits the water
a) his linear momentum is constant
*b) his angular momentum is constant
c) his angular velocity is constant
d) all of the above
e) none of the above
- 6.
- A car going over a bump starts vibrating at a
frequency of 4 oscillations/second. If you put on the car roof an elephant
whose weight is equal to that of the car + driver, when going over the same
bump the car will vibrate at
a) 2 oscillations/s
*b) 2.8 oscillations/s
c) 4 oscillations/s
d) 5.6 oscillations/s
e) 8 oscillations/s
- 7.
- Water is a good storage for thermal energy since
it has
a) low density
*b) high heat capacity
c) 00C freezing point
d) anomalous heat expansion behaviour at 40C
e) none of the above
- 8.
- A mixture of Oxygen-16 and Nitrogen-14 is in thermal
equilibrium. If the rms (root mean square) speed of the Oxygen molecules is
200 m/s, what is the Nitrogen molecules rms speed? (both molecules are
di-atomic)
a) 228 m/s
b) 175 m/s
c) 187 m/s
*d) 214 m/s
e) 374 m/s
- 9.
- You can't believe your luck when, at an auction, you
buy an apparently solid gold 1.5 kg paperweight for a few dollars ! When at
home, you lower it into a beaker of water and observe that the increase in
water level corresponds to a displaced volume of 100 cm3. Knowing that
the density of gold is 1.93 x 104 kg/m3, you conclude that
a) yes, you made a great purchase
*b) well, it cannot be solid gold, but it does look nice anyway
c) the density is larger than gold, something must be wrong
d) the procedure does not provide enough information to reach any conclusion
e) none of the above
- 10.
- A virus is known to require temperatures exceeding
1000C in order to be completely destroyed. Which of the following
techniques would not work to remove the virus from contaminated material ?
a) boil it in a pressure cooker
b) boil it in heavily salted water
*c) boil it in a regular pot until all the water evaporates
d) drop it in molten lead (melting point 3270C)
e) they would all work
- 11.
- An aluminum cube painted white is exposed to
sunlight. If its temperature is about 600C
a) it reflects and emits radiation that are both visible
b) emitted radiation is visible, reflected is not
*c) reflected radiation is visible, emitted is not
d) neither reflected nor emitted radiation are visible
e) not enough information
- 12.
- When stars run out of nuclear fuel, eventually they
collapse into incredibly dense objects called neutron stars. Suppose that a
sun-sized star (radius = 7 x 108 m) collapses into a 7 km radius neutron
star. If the star's rotational period before collapse was 107 seconds (about
116 days), what will be the neutron star's rotational period ?
*a) 1 ms
b) 2 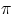 ms
ms
c) 10-7 s
d) 1 s
e) 1/(2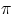 ) ms
) ms
- 13.
- What is the density of carbon dioxide (CO2,
molecular mass 44) at Standard Temperature and Pressure (P = 1 atm, T =
00C) ?
*a) 1.96 kg/m3
b) 3.72 kg/m3
c) 5.03 kg/m3
d) 0.22 kg/m3
e) 7.28 kg/m3
- 14.
- A 12 kg mass attached to a spring is moved 0.4 m
away from the position of equilibrium and then released. If the spring constant
is 18 N/m, what is the mass maximum acceleration ?
a) 2.67 m/s2
b) 1.50 m/s2
c) 0.33 m/s2
d) 0.49 m/s2
*e) 0.60 m/s2
- 15.
- In your car radiator heat transfer occurs primarily
via
a) radiation
b) conduction
*c) forced convection
d) unforced convection
e) phase change
- 16.
- You are told that your systolic blood pressure is
120 mm Hg. This means that
a) the weight of your blood is enough to sustain a 120 mm column of mercury
b) your blood has the same average density as a column of 120 mm of mercury
c) the maximum absolute pressure exerted by the heart is equivalent to the
pressure exerted by a column of 120 mm of mercury
*d) the maximum pressure exerted by the heart exceeds atmospheric pressure
by an amount equivalent to the pressure of a 120 mm column of mercury
e) the velocity at which your blood moves induces a pressure differential of
120 mm of mercury
- 17.
- When the water is turned on, a two-jet rotating water
sprinkler reaches an angular velocity of 5 rad/s in one second. If you
approximate the sprinkler as a 200 gram thin rod 40 cm long pivoting around
its center, what is the torque exerted by each of the two jets ?
*a) 6.7 x 10-3 N m
b) 13.3 x 10-3 N m
c) 26.7 x 10-3 N m
d) 53.3 x 10-3 N m
e) 106.7 x 10-3 N m
- 18.
- Your car windshield is covered with a 1 mm thick
layer of ice over a 1.5 m2 area. If the ice temperature is at -100C,
for how long do you need to run a 1500 watt hair-drier in order to melt it
completely?
*a) about 5 and a half minutes
b) 90 seconds
c) 42 seconds
*d) about 6 and a half minutes
e) about 1 minute
(note: two solutions are allowed, depending on whether you use the correct value
for ice heat capacity, or, as I had originally in mind, whether you just use the
water value of 1)
- 19.
- An adult and a child are sitting on adjacent
identical swings. Once they get moving the adult will swing with
a) much greater period
b) much greater frequency
c) exactly the same period
*d) the same amplitude
e) none of the above
- 20.
- When you sweat, the process mainly responsible for
taking heat away from your body is
a) radiation
b) convection
c) forced convection
d) conduction
*e) phase change
- 21.
- To balance the weight of a 50 kg St. Bernard dog, a
girl uses a 12 meter long plank as a lever. If the maximum force she can apply
is 100 N, the pivoting point must be
a) 4 meters from the girl
*b) 10 meters from the girl
c) 8 meters from the dog
d) 4 meters from the dog
e) none of these
- 22.
- A sample of Oxygen gas (molecular mass = 32) is kept
in a cylinder with a movable piston. If the piston is moved in such a way that
the volume is doubled and the pressure is halved, and the sample initial
temperature is 100C, what is the final temperature?
a) 50C
b) 200C
c) 566 K
d) 141.5 K
*e) 283 K
- 23.
- When an ideal fluid flowing steadily through a
conduit encounters a bottleneck
a) both speed and pressure increase
b) both speed and pressure decrease
*c) speed increases and pressure decreases
d) speed decreases and pressure increases
e) not enough information
- 24.
- In a harmonic motion, position, velocity and
acceleration have all the same sign
a) when going from x = xmax to x=0
b) when going from x = 0 to x = -xmax
c) when going from x = -xmax to x=0
d) when going from x = 0 to x = xmax
*e) never
- 25.
- A 2 kg sample of unkown material is heated to 800C
and thrown into 1 kg of water at 240C. Equilibrium is reached at a
temperature of 300C. What was the material? (Specific heat capacities, in
Cal/(kg 0C): Al 0.215, Cu 0.09, Fe 0.11, Pb 0.03, Ag 0.06)
a) Aluminum
b) Copper
c) Iron
d) Lead
*e) Silver



Next: About this document ...
Sergio Conetti
12/10/1999