


Next: About this document ...
Chapter 19
Temperature
Temperature is a concept with which we are very familiar, even though it involves a certain amount of
subjectivity (ice cream at room temperature is warm, while room temperature coffee is cold). In Physics,
we need to define temperature in a somewhat more rigorous way. Soon we will find out that what we perceive as
temperature of an object is a macroscopic manifestation of the motion of the object's molecules, and in fact we
will see that temperature is proportional to the average kinetic energy of the molecules. But for now we will
not consider the molecular motion but we will introduce the idea of temperature in the following way:
- take two objects, a "cold" one and a "hot" one and put them in contact with each other. As experience
tells us, after a while the cold one will warm up and the hot one cool down
- this process occurs since there is a transfer of (thermal) energy from the hot one to the cold one
- after a while, the objects are in thermal equilibrium, i.e no net energy transfer
occurs any more
- when two objects are in thermal equilibrium , we say that they are at the same temperature. Conversely,
we could say that temperature is the property that determines whether two objects are in thermal equilibrium.
Note that, in order for the whole definition and relative comparison of temperatures to make sense, we need
to assume the validity of the zeroth law of Thermodynamics
if both objects A and B are in thermal equilibrium with a third object C, then A is in thermal equilibrium
with B
From a practical point of view, we can assign a (relative) numerical value to temperatures by exploiting one
of many temperature-dependent properties of matter, viz.
- 1.
- the length of a thin rod (or any dimension of a solid)
- 2.
- the volume of a liquid
- 3.
- the volume of a gas (at constant pressure)
- 4.
- the pressure of a gas (at constant volume)
- 5.
- the electrical resistance of a conductor
- 6.
- the colour of an object (more precisely, the spectrum of electro-magnetic radiation emitted by an
object)
We can exploit property 4 to realize that temperature values are not completely relative, i.e. depending on our
arbitrary choice of temperature scales, but are absolute, at least in the sense that there is a lowest
possible temperature, the absolute zero. Experimentally, we could measure the pressure
of a gas kept at constant volume while varying the temperature, and notice that there is a simple
proportionality between temperature (measured e.g. in 0C with a mercury thermometer) and pressure
(measured with any arbitrary barometer or manometer). The (pressure vs. temperature) curve is then a straight line
P = m TC + q
showing that P=0 for TC=-q/m . We can already guess that such a temperature is the lowest one
could get, since below that value one would have (meaningless) negative pressure. Moreover, we could see
that any other gas behaves the same way, i.e. its pressure s. temperature behaviour is given by
P = m' TC + q'
with q/m = q'/m' = 273.15 0C. This temperature is what is called the absolute zero. As mentioned
before, we will eventually relate temperature to molecular motion, therefore it would be appealing to think of
the absolute zero as the temperature where all the molecules are at rest. This picture, while meaningful in
classical physics, is not valid within the realm of Quantum Mechanics. Still, it is legitimate to sate that
absolute zero corresponds to the state where all elementary constituents are at their lowest possible energy
level. Abolute temperatures are measured in kelvins, TK = TC + 273.15 .
Thermal Expansion Most substances (with some notable exceptions) increase their dimensions at the
increasing of temperatures. Quantitatively, the growth 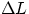 in a given direction for a temperature
change
in a given direction for a temperature
change 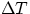 can be expressed by
where
can be expressed by
where 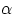 is the coefficient of linear expansion. Note that, as long as one is dealing with
temperature differences,
is the coefficient of linear expansion. Note that, as long as one is dealing with
temperature differences, 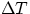 has the same value whether measured in kelvins or celsius.
A similar expression is valid to represent changes in volume
and, typically,
has the same value whether measured in kelvins or celsius.
A similar expression is valid to represent changes in volume
and, typically, 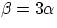 (you can easily prove this by considering the growth in volume in a cube of
side L, and neglecting terms containing
(you can easily prove this by considering the growth in volume in a cube of
side L, and neglecting terms containing 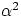 and
and 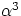 ).
).
Water has a (very important) anomalous behaviour, since its volume decreases with decrasing temperature down
to 40C, at which point it starts increasing again (this is why ice floats and large bodies of water only
freeze at the surface).
Ideal Gas Equation of State
We have already mentioned the proportionality between pressure and temperature (at constant volume), as well as
of volume and temperature (at constant pressure). These two properties can be summarized as
PV = nRT
wehre T is the absolute temperature, n is the number of moles in the gas sample under exam and R is a
universal constant, i.e a constant valid for any ideal gas . In SI units,
R=8.315 J/(mol K). What is an ideal gas? We could cheat and say that an ideal gas is a gas for which the
PV=nRT expression is
valid.... More usefully, a real gas will to a very good extent behave like an ideal gas if it is far enough
from its condensation point, and the (infrequent) interactions among molecules can be interpreted purely in
terms of billiard ball-like elastic collisions.
Reminder : what is a mole? The definition given in the book is quite convenient : a mole of a substance is
an amount containing 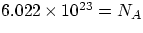 particles (atoms or molecules) of the substance (NA=
Avogadro's number). You might have
encountered different definitions, e.g. " a mole is an amount in grams numerically equal to the molar
mass of the substance", or " one mole is the number of atoms in a 12 gram sample of Carbon-12". All these
definitions are equivalent, due to the fact that one mole of a substance always contains the same number
NA of particles (atoms or molecules). If N is the total number of particles in a sample, we also have
particles (atoms or molecules) of the substance (NA=
Avogadro's number). You might have
encountered different definitions, e.g. " a mole is an amount in grams numerically equal to the molar
mass of the substance", or " one mole is the number of atoms in a 12 gram sample of Carbon-12". All these
definitions are equivalent, due to the fact that one mole of a substance always contains the same number
NA of particles (atoms or molecules). If N is the total number of particles in a sample, we also have
n = N/NA
Moreover, if Mm is the molar mass (i.e. the mass of one mole) of our substance, and m the mass of one
molecule,
Mm = mNA
Finally, if M is the total mass of the sample
n = M/Mm=M/(mNA)



Next: About this document ...
Sergio Conetti
11/29/2000