


Next: Problem 17.30
Up: No Title
Previous: Problem 17.23
17.25. a)
The net thermal energy transferred to the gas is equal to
the work done by the system. The work done by the system is the area
under the work pressure vs. volume graph. If the process is cyclic,
then the work done is equal to the area of the enclosed region.
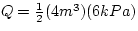
Q = 12.0 kJ
b)
If the process is reversed, then work done is the negative of what we
found in part a. In other words, the area under AB is larger than the
area under AC and if the process is reversed from the drawing in the
book, then the work done during AB is negative and the work done in AC
is positive. Therefore,
Q = -12.0 kJ
Jason George Zeibel
12/10/1997