Angular Momentum Quantization: Physical Manifestations and Chemical Consequences
Michael Fowler
University of Virginia
Physics 252 Home Page
Link to Previous Lecture
The Stern-Gerlach Experiment
We’ve established that for the hydrogen atom, the angular momentum of the electron’s orbital motion has values ![]() , where l = 0, 1, 2, … , and the component of angular momentum in the z-direction is
, where l = 0, 1, 2, … , and the component of angular momentum in the z-direction is ![]() , where m takes integer values - l, - l + 1, … , +l. This means that if we measure the angle between the total angular momentum and the z-axis, there can only be 2l + 1 possible answers, the total angular momentum cannot point in an arbitrary direction relative to the z-axis, odd though this conclusion seems. This is sometimes called "space quantization".
, where m takes integer values - l, - l + 1, … , +l. This means that if we measure the angle between the total angular momentum and the z-axis, there can only be 2l + 1 possible answers, the total angular momentum cannot point in an arbitrary direction relative to the z-axis, odd though this conclusion seems. This is sometimes called "space quantization".
Is there any way we can actually see some effect of this directional quantization? The answer is yes—because the electron moving around its orbit is a tiny loop of electric current, and, therefore, an electromagnet. Therefore, if we switch on a magnetic field in the direction of the z-axis, the energy of the atom will depend on the degree of alignment of its magnetic moment with the external applied magnetic field. The magnetic field from a small current loop is like that from a small bar magnet aligned along the axis of the loop. The simplest way to see how the potential energy of the little magnet depends on which way it’s pointing relative to the field is to take a little bar with an N pole of strength +p at one end, an S pole of strength -p at the other end. Think of a compass needle, of length d, say. The magnetic moment is defined as pole strength multiplied by distance between the poles, m = pd, and is considered to be a vector pointing along the axis of the magnet, from S to N. The potential energy of this little magnet in an external field H is -m .H, lowest when the magnet is fully aligned with the field. It is easy to check this: counting as zero potential energy the magnet at right angles with the field, the work needed to point it at an angle q is 2.p.d/2.cosq .
The magnetic moment of a current I going in a circle around an area A is just IA. The electron has charge e, and speed v, so goes around v/2p r times per second. In other words, if you stand at one point in the orbit, the total charge passing you per second is ev/2p r = I. Hence the magnetic moment, usually denoted m L = IA, is p r2.ev/2p r = rev/2. The angular momentum is L = mvr, so
m
L = eL/2m.Thus if the electron is in an l = 1 orbit, the current will generate a magnetic moment ![]() , which is 9.3 ´ 10-24 joules per tesla, or 5.8 ´ 10-5 eV per tesla. Note that this means in a one tesla field an atomic energy level will move ~10-4 eV, an easily detectable shift in spectral lines will result.
, which is 9.3 ´ 10-24 joules per tesla, or 5.8 ´ 10-5 eV per tesla. Note that this means in a one tesla field an atomic energy level will move ~10-4 eV, an easily detectable shift in spectral lines will result.
But there is a more direct way to see how the atoms are oriented, the Stern Gerlach apparatus. In this experiment, a beam of atoms is sent into a nonuniform magnetic field. This means the north and south poles of a small bar magnet would feel different strength forces, so there would be a net force on a small magnet, and hence on an atom. Furthermore, the direction of this force would depend on the orientation of the dipole. Suppose the nonuniform field is pointing upwards, and is stronger at the top. Then a small bar magnet oriented vertically with the north pole on top will be pushed upwards, because the north pole will be experiencing the stronger force. If the south pole is on top, the magnet will be pushed downwards. If the magnet is horizontal, there will be no net force (assuming magnetic field strength varies only negligibly in the horizontal direction).
Imagine, then, a stream of atoms with magnetic moments entering a region of magnetic field as described. Each atom will feel a vertical force depending on the orientation of its magnetic moment. If with no magnetic field present the stream of atoms formed a dot on a screen after passing through the apparatus, on switching on the field one would expect the dot to be stretched into a vertical line, if one assumed equal likelihood of all orientations of the magnetic moment. However, the quantum theory predicts that this is not the case—we have argued that for l = 1, say, there are only three allowed orientations of the magnet (atom) relative to the field. Therefore, we would predict that three dots (or, more realistically, blobs) would appear on the screen, not a continuous line.
In fact, when the experiment was carried out, there was a very surprising result. The most dramatic form of the new result came in 1927, when ground state l = 0 hydrogen atoms were used. Such atoms have no orbital angular momentum, and therefore no orbital current, and were not expected to show magnetic effects. Yet on going through the Stern-Gerlach apparatus, the beam of hydrogen atoms split into two! This was difficult to interpret, because the least allowed angular momentum, l = 1, would give three blobs, and l = 0 would give only one. You might expect a mixture to give one strong blob and two weak ones, but two equal blobs didn’t seem possible, theoretically.
The solution to the problem was suggested by two graduate students, Goudsmit and Uhlenbeck. They suggested that the electron itself had a spin. That is to say, in a general orbit, the electron both orbited the proton and spun on its own axis, just as the earth orbits the sun once a year and also spins on its own axis once a day. If the electron spin is assumed to be ![]() , and we assume as before that the z-component can only change by whole units of
, and we assume as before that the z-component can only change by whole units of ![]() , then there are only two allowed values of the z-component,
, then there are only two allowed values of the z-component, ![]() . Of course, this is a hand waving argument—the reason the z-component only changed by integers was that the wave function had to fit a whole number of wavelengths on going around the z-axis. But our wave function for spin one-half, if it is of the same form as those for angular momentum, must have a term eij /2, and so is multiplied by -1 on rotating through 2p !
. Of course, this is a hand waving argument—the reason the z-component only changed by integers was that the wave function had to fit a whole number of wavelengths on going around the z-axis. But our wave function for spin one-half, if it is of the same form as those for angular momentum, must have a term eij /2, and so is multiplied by -1 on rotating through 2p !
Further difficulties arose when people tried to construct models of how a spinning electron would have its own magnetic moment. It’s not too difficult to see how this might occur—if the electron is a charged sphere, or has charge on its surface, then its rotation implies that this charge is going around in circles, little current loops, and so will give a magnetic field. The problem was, it was known that the electron was a very small object. It turned out that the equatorial speed of the electron would have to be greater than the speed of light for the magnetic moment to be of the observed strength.
These difficulties in understanding the electron spin and magnetic moment were far from trivial, and in fact were not resolved until around 1930, by Dirac, who gave a fully relativistic treatment of the problem, which, remarkably, predicted the magnetic moment correctly and at the same time treated the electron as a point particle. There is no simple picture presenting this in classical or semiclassical terms, but Dirac’s work is the basis of our modern understanding of particle physics. It is unfortunately beyond the scope of this course.
The bottom line, as far as we are concerned, is that assuming the electron has spin one-half and hence two possible spin orientations with respect to a given axis explains the observed Stern-Gerlach results, and also, more importantly, helps us construct the periodic table, as we shall see below.
Building the Periodic Table
The atomic number (usually denoted Z) of an element denotes its place in the periodic table, so H has Z = 1; He, Z = 2; Li = 3, Be = 4, B = 5, C = 6, N = 7, O = 8, F = 9, Ne = 10, and so on. This number is equal to the number of protons in the nucleus, and also equal to the number of electrons orbiting around the nucleus, to preserve electrical neutrality.
To try to understand how the electrons orbit the nucleus, we need to make some simplifying assumptions. We are not going to be able to solve Schrödinger’s equation for even two electrons exactly, if we include their repulsion of each other. However, the presence of the other electrons is clearly important—their repulsion to some extent counteracts the attraction the nucleus has for a given electron. For an electron imagined to be in some outer orbit, the electrons in closer to the nucleus orbits lower the effective nuclear charge. Thinking now about the force felt by one electron, a simple approximation is to imagine all the other electrons as changing the electrical attraction the one electron feels from the nucleus to a shielded attraction, so that the further it is away from the nucleus, the weaker an attractive charge it sees.
We then make the naïve assumption that all the electrons see the same potential, this shielded Coulomb potential, so we have Z electrons all in the same potential well, but we assume they are independent particles, in the sense that they do not repel each other, except to the extent already taken into account by changing to a shielded potential. So the question is, what are the possible wave functions of Z independent electrons in this well? The crucial point is that although they do not interact with each other, they are identical, so the wave function must be antisymmetrized, as we discussed for the two-particle case earlier. This means that the electrons must be in different bound states in the well—the Pauli exclusion principle. But what do the bound state wave functions look like in this potential? Since the shielded Coulomb potential is still spherically symmetric, all our arguments about the q , j behavior of the ordinary Coulomb potential apply equally to the shielded case, in particular the angular momentum has values ![]() , where l = 0, 1, 2, …, and the component of angular momentum in the z-direction is
, where l = 0, 1, 2, …, and the component of angular momentum in the z-direction is ![]() , where m takes integer values - l, - l + 1, … , +l. Furthermore, each electron has spin
, where m takes integer values - l, - l + 1, … , +l. Furthermore, each electron has spin ![]() , and there are two allowed values of the spin z-component,
, and there are two allowed values of the spin z-component, ![]() . The radial wave functions R(r) are clearly somewhat different from those in the pure Coulomb case. The main difference is that states of different angular momentum which were degenerate in the Coulomb case are no longer the same energy in the shielded Coulomb case. If you examine wave functions corresponding to the same energy but different values of l, you will see that the higher the l-value, the smaller the wave function is near the nucleus. This means the higher l wave functions do not feel the powerful unshielded potential near the nucleus, and so are not as strongly bound as the lower l functions.
. The radial wave functions R(r) are clearly somewhat different from those in the pure Coulomb case. The main difference is that states of different angular momentum which were degenerate in the Coulomb case are no longer the same energy in the shielded Coulomb case. If you examine wave functions corresponding to the same energy but different values of l, you will see that the higher the l-value, the smaller the wave function is near the nucleus. This means the higher l wave functions do not feel the powerful unshielded potential near the nucleus, and so are not as strongly bound as the lower l functions.
Notation
Atomic physicists use a standard notation to enumerate these states. The different angular momenta are denoted by letters, s for l = 0, p for l = 1, d for l = 2, f for l = 3, g for l = 4 and then on alphabetically. The principal quantum number n, such that for the hydrogen atom E = -1/n2 in Rydberg units, is given as a number, so the lowest hydrogen atom state is written 1s. The two n = 2 orbital states are 2s and 2p, then come 3s, 3p and 3d and so on. From the discussion immediately above, 2s and 2p have the same energy in the hydrogen atom, but for the shielded potential used to approximate for the presence of other electrons in bigger atoms 2s would be more tightly bound, and so at a lower energy, than 2p.
Filling an Atom with Electrons
Let us now consider taking a bare nucleus, charge Z, and adding Z electrons to it one by one. From the Pauli exclusion principle, each electron must be in a different state. But remember that having a different spin counts as different (you could tell them apart) so we can put two electrons, with opposite spins, into each orbital state. Thus He has two electrons in the 1s state. Li must have two electrons in 1s, and one electron in 2s. This suggests a picture of one electron outside of a "closed shell" of two 1s electrons. The next occurrence of a similar picture is Na, Z = 11, which is chemically very similar to Li. This means that 10 electrons fill closed shells. We can understand this because 2 go into 1s, 2 go into 2s and 6 fill 2p. But notice by saying it takes 6 electrons to fill 3p, we are saying there are three distinct l = 1 orbitals. In other words, the chemical properties of the elements support and confirm the hypothesis of "space quantization"—that there are only three distinct l = 1 angular wave functions, those given by m = 1, 0 and -1.
Atoms interact chemically by sharing or partially transferring electrons. Not surprisingly, atoms with filled shells only, like He and Ne, are chemically unreactive. The valency, roughly speaking, is the number of electrons available for transfer (so Li and Na have valency 1) or available sites for reception of electrons—fluorine has an outer shell with one vacancy, so a valency of 1. To some extent, valency can vary depending on the strength of attraction of other atoms in the chemical environment.
Filling a Box with Electrons
When many Li atoms are put together to form a solid, it is found that the loosely attached outer electrons leave their original atoms and wander freely throughout the metal. Their wave functions are well represented by standing plane waves in a box (let’s take a cube of metal, of side L). Each such plane wave state in the box can be represented by three numbers nx, ny and nz, representing the number of nodes of the standing wave in the x, y and z directions respectively. Extending slightly our analysis of an electron in a two-dimensional box, the energy of such a state will be 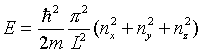 . Thus if we imagine pouring electrons into an empty lattice of Li atoms each with one electron missing(not a physically realistic procedure!) two electrons (opposite spins) will go into each state, first (0, 0, 0) then (1, 0, 0) or equally (0, 1, 0) etc., and from the form of the energy we can see that in (nx, ny, nz) space, the electrons will fill up all the positive integer points within a sphere up to some maximum energy determined by how many electrons we put in. Notice that since the n’s are all positive integers, the filled space is only the one-eighth of the sphere’s volume corresponding to nx > 0, ny > 0 and nz > 0 for the sphere centered at the origin.
. Thus if we imagine pouring electrons into an empty lattice of Li atoms each with one electron missing(not a physically realistic procedure!) two electrons (opposite spins) will go into each state, first (0, 0, 0) then (1, 0, 0) or equally (0, 1, 0) etc., and from the form of the energy we can see that in (nx, ny, nz) space, the electrons will fill up all the positive integer points within a sphere up to some maximum energy determined by how many electrons we put in. Notice that since the n’s are all positive integers, the filled space is only the one-eighth of the sphere’s volume corresponding to nx > 0, ny > 0 and nz > 0 for the sphere centered at the origin.
Physicists sometimes formulate this filling of electron states slightly differently, by imposing periodic boundary conditions on a piece of metal, the three-dimensional generalization of replacing a finite line by a ring, so each electron can go round and round in the same direction indefinitely. This is not easy to actually do in three dimensions, but is convenient to talk about, and in fact taking different boundary conditions has a very tiny effect on the bulk electronic properties of metals -- there are just so many electrons in an ordinary-sized piece of metal. The advantage of using periodic boundary conditions is that instead of standing waves (which can be thought of as representing electrons bouncing back and forth), each electron now has a definite momentum. The allowed momenta form a grid in "momentum space" a lot like the allowed integers in the standing waves above. In fact it turns out that there are the same number of allowed momenta up to a given energy as there are allowed standing wave states (for a large system). The difference is that in momentum space, if momentum k is allowed, so is - k, and in the ground state momentum states are filled up to a spherical surface, called the "Fermi Surface". This surface can be visualized as an energy equipotential in "momentum space" at the "Fermi energy". Typical Fermi energies are of the order of electron volts. By spreading out through the metal in this way the electrons attain an overall lower energy state than if each stayed with its own atom. This is why the solid is stable. On applying heat to the electrons, even 1000K is only 0.1eV, so only those near the surface of the filled sphere are free to move, because of the exclusion principle, the others are locked in. This means the heat capacity of the electrons is much less than (3/2)kT per particle, as would be predicted classically. This was another long standing classical puzzle solved by the advent of quantum mechanics.
Copyright Ó 1997 Michael Fowler