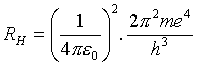
From Bohr's Atom to Electron Waves
Michael Fowler
University of Virginia
Physics 252 Home Page
Link to Previous Lecture
Reactions to Bohr's Model
Bohr's interpretation of the Balmer formula in terms of quantized angular momentum was certainly impressive, but his atomic model didn't make much mechanical sense, as he himself conceded. For example, an electron jumping from the nth orbit to the mth emitted radiation at frequency equal to the energy difference of the orbits divided by h. Presumably, it began radiating as soon as it left its original orbit. As Rutherford put it in a letter to Bohr, "how does an electron decide what frequency it is going to vibrate at when it passes from one stationary state to another? It seems to me that you would have to assume that the electron knows beforehand where it is going to stop."
A few days later, Bohr sent Rutherford another manuscript containing the correspondence principle argument gone over in our last lecture. This was a bit too long for Rutherford. He responded: "As you know, it is the custom in England to put things very shortly and tersely in contrast to the Germanic method, where it appears to be a virtue to be as long-winded as possible".
The Germans themselves were very skeptical of Bohr's model. Harald Bohr wrote to his brother from Göttingen in the fall of 1913 that the young physicists there considered Bohr's model too "bold" and "fantastic".
Mysterious Spectral Lines
One good reason for the Germanic skepticism was the recent discovery of apparently new spectral lines for hydrogen corresponding to half-integers in the Balmer formula. These new lines had been seen by a spectroscopist, Alfred Fowler, in a discharge tube containing a mixture of hydrogen and helium, and also in the spectra of a star by Pickering.
When he heard about these new lines, Bohr realized that his formula for the Rydberg constant,
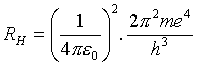
should also apply to the ionized helium atom -- that is, a helium nucleus with a single electron in orbit around it, provided e2 is replaced by 2e2, to correct for the doubly charged nucleus. This gives an overall factor of 4, exactly equivalent to replacing the integers in the denominator by half-integers. In other words, Bohr argued, the supposed new hydrogen lines in fact were from ionized helium.
Fowler, however, was a very precise experimentalist. He could measure the spectral lines to five significant figures. Therefore, despite uncertainties in the values of e, m and h, the ratio of the "Rydberg constant" for helium to that for hydrogen could be measured to great accuracy. Fowler found it wasn't 4, but actually 4.0016.
Bohr's response to this news was conclusive. He pointed out that his analysis of the circular motion had neglected the finite mass of the nucleus. He should really have taken the electron to have an effective mass equal to mM/(m + M). It is easy to verify that if this correction is made for both hydrogen and helium, the ratio changes from 4 to 4.0016!
This discovery won over most physicists to the point of view that Bohr was definitely on to something. Sir James Jeans remarked that the only justification for Bohr's postulates was "the very weighty one of success". According to Rosenfeld (Introduction to Niels Bohr Collected Works): "At Göttingen, that high place of mathematics and physics, where the sense for propriety was strong, the prevailing impression was one of scandal, or at least bewilderment, before the undeserved success of such high-handed disregard of the canons of formal logic." But all Germans didn't feel that way. Bohr's friend George Hevesy told Einstein about Bohr's identification of the Pickering-Fowler lines with helium. Einstein said: "This is an enormous achievement. The theory of Bohr must then be right."
A Periodic Table Puzzle and the X-ray Connection
Mendeleev's success in demonstrating that the elements had recurring patterns of chemical behavior when arranged in order of increasing atomic weight had some anomalies. In particular, cobalt has a higher atomic weight than nickel, yet its chemical properties strongly suggested it should come before nickel in the table. It was gradually realized over the period from 1907 to 1913 (primarily by a Dutchman, van den Broek, actually a real estate lawyer who did physics in his spare time (Pais page 227)) that the significant parameter from a chemical point of view was not the mass number A, but the total number of electrons, and hence the charge on the nucleus Z, once the nuclear model was accepted.
The problem was measuring the nuclear charge. This could in fact be done by Rutherford scattering, but that was a long and tedious process. X-rays gave a quicker way. As we said in the last lecture, any substance on being bombarded with sufficiently fast electrons emits a continuum of x-ray frequencies up to a maximum frequency f given by hf = kinetic energy of electron, plus some sharply defined lines. The frequency of the sharply defined lines gradually increases with atomic number.
These x-ray lines were first discovered by Max von Laue in Munich in the summer of 1912. Harry Moseley, who worked with Rutherford mostly teaching undergraduates, decided to investigate this new spectroscopy. When Bohr came to visit in 1913, he encouraged Moseley to investigate x-ray spectra of many elements with a view to pinning down the values of Z. Moseley used a cathode ray tube a yard long with a little train inside it having a different element in each car, so many readings could be taken in one run (Pais page 229). He found several different x-ray lines for each element, with very similar patterns for each element, although gradually shifting to higher frequencies with increasing Z. For example, one particular line, labeled Ka , had a Z-dependent frequency:
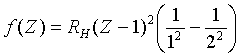
This made it possible to order all the elements unambiguously. It was also good evidence that Bohr's theory was close to correct for the innermost electrons in the atom, those in the field of the unshielded nucleus. Actually, the appearance of Z - 1 rather than Z in the formula is because the innermost shell has just two electrons, as we shall see later, and they partially shield the nuclear charge from each other. The appearance of this factor was not understood at the time, because it was thought that there were more electrons in the innermost shell.
Bohr later remarked that Moseley's extremely clear x-ray work had far more impact than Rutherford's scattering experiment in widening acceptance of the Bohr-Rutherford atom. Tragically, Moseley died two years later, killed by a bullet in the head at Gallipoli.
What Bohr's Model Achieved
As Bohr himself emphasized, his model atom didn't explain anything, in the way classical dynamics or electromagnetism could explain how systems functioned in terms of a few underlying laws. What the Bohr model did do was to tie together previously unrelated phenomena, and therefore clarify the agenda for future investigations. It showed there was a link between the Balmer formula, Planck's constant and the nuclear atom, with quantized angular momentum playing a central role. The precision of the spectral predictions for ionized helium demonstrated something was right about the model, as did the interpretation of characteristic x-rays for many elements in terms of inner-shell electrons. On the other hand, despite heroic efforts over several years by Bohr and others, there was no successful prediction of the observed spectral lines for the next simplest atom -- neutral helium. Evidently, to make further progress in understanding atoms, some new insight was needed.
At this point the war intervened. Much of the momentum in physics was lost. Rutherford's lab was practically emptied, Rutherford himself spent most of his time on antisubmarine research. Bohr put much effort into establishing a physics institute in Copenhagen. He also thought at length about how the periodic table might be understood in terms of filling an atom with electrons. There was something of a breakthrough in 1924 when the German physicist Wolfgang Pauli suggested there must be a law that two electrons couldn't go into the same orbit. (By this point, the number of allowed orbits had been substantially increased by Sommerfeld to include Keplerian elliptic ones.) Pauli's "exclusion principle" was introduced as an empirical rule. We shall see later that it follows very naturally from symmetry considerations. We shall also find that in the full quantum picture of the atom, although the angular momentum is quantized, as Bohr realized, the orbital angular momentum is actually zero in the ground state of the hydrogen atom. One of the more surprising results of the full quantum theory is that the energy levels found by Bohr, and even relativistic corrections computed by Sommerfeld, are exactly correct, even though the model of classical inverse square orbits is far from the truth (except for very large orbits) as we shall see.
A Student Prince Catches a Wave
Despite all the effort in Copenhagen and Göttingen, the next real advance in understanding the atom came from an unlikely quarter -- a student prince in Paris. Prince Louis de Broglie was a member of an illustrious family, prominent in politics and the military since the 1600's. Louis began his university studies with history, but his elder brother Maurice studied x-rays in his own laboratory, and Louis became interested in physics. He worked with the very new radio telegraphy during the war.
After the war, de Broglie focused his attention on Einstein's two major achievements, the theory of special relativity and the quantization of light waves. He wondered if there could be some connection between them. Perhaps the quantum of radiation really should be thought of as a particle. If so, the theory of special relativity suggested that the observed energy-momentum relationship, E = cp, meant that it had a very small rest mass, for it was always observed -- naturally -- to be moving at the speed of light. De Broglie suspected that if the speed of a sufficiently low energy quantum could be measured, it would be found to be less than c. On this point he was wrong (as far as we know!). Nevertheless, it was a very valuable conceptual breakthrough to think of the quantum of radiation as a particle, knowing full well that radiation is a wave. In fact, his incorrect idea that the photon (as we now call the light quantum) had a rest mass led him to analyze the relationship between particle properties and wave properties by transforming to the rest frame of the photon, and he discovered that the energy and momentum of the particle were related to the frequency and wavelength of the wave by:
E = hf, p = h/l
Of course, the first condition is the Planck-Einstein quantization, and the second follows trivially from it if we take E = cp and l f = c. But de Broglie showed it was more generally true -- it worked even if the photon had a rest mass.
Having decided that the photon might well be a particle with a rest mass, even if very small, it dawned on de Broglie that in other respects it might not be too different from other particles, especially the very light electron. In particular, maybe the electron also had an associated wave. The obvious objection was that if the electron was wavelike, why had no diffraction or interference effects been observed? But there was an answer. If de Broglie's relation between momentum and wavelength, p = h/l , also held for electrons, the wavelength was sufficiently short that these effects would be easy to miss. As de Broglie himself pointed out, the wave nature of light isn't very evident in everyday life, or in ray tracing in geometrical optics. He suspected the apparently pure particle nature of electronic trajectories was analogous to the apparent straight line propagation of rays of light, over distance scales much greater than the wavelength.
However, the wavelike properties should be important on an atomic scale. No progress had been made in a decade in understanding why the electronic orbits in the Bohr atom were restricted to integral values of the angular momentum in units of h. But if the electron were in some sense a wave, it would be very natural to restrict the orbits to those of standing waves, for otherwise the electron wave on going around the orbit would interfere with itself destructively.
Suppose now the electron, having momentum p, is moving in a circular orbit of radius r. Then for a standing wave, a whole number of wavelengths must fit around the circle, so for some integer n, nl = 2p r. Putting this together with p = h/l , we find:
2p r = nl = nh/p
so
L = pr = nh/2p.
The "standing wave" condition immediately gives Bohr's quantization of angular momentum!
This was the prince's Ph. D. thesis, presented in 1924. His thesis advisor was somewhat taken aback, and wasn't sure if this was sound work. He asked de Broglie for an extra copy of the thesis, which he sent to Einstein. Einstein wrote shortly afterwards: "I believe it is a first feeble ray of light on this worst of our physics enigmas". The prince got his Ph. D.
An Accident at the Phone Company Makes Everything Crystal Clear
There was an accident at the Bell Telephone Laboratories in April 1925. Clinton Davisson and L. H. Germer, looking for ways to improve vacuum tubes, were watching hoe electrons from an electron gun in a vacuum tube scattered off a flat nickel surface. Suddenly, while the experiment was running and the nickel target was very hot, a bottle of liquid air near the apparatus exploded, smashing one of the vacuum pipes, and air rushed into the apparatus. The hot nickel target oxidized immediately. The layer of oxide made their target useless for further investigations. They decided to clean off the oxide by heating the nickel in a hydrogen atmosphere then in vacuum. After doing this for a prolonged period, the nickel looked good, and they resumed the investigation.
To their amazement, the pattern of electron scattering from the newly cleaned nickel target was completely different from that before the accident. What had changed? On examining their newly cleaned crystal carefully, they found a clue. The original target was polycrystalline -- made up of a multitude of tiny crystals, oriented randomly. During the prolonged heating of the cleaning process, the nickel had re-crystallized into a few large crystals.
To quote from their paper: "It seemed probable to us from these results that the intensity of scattering from a single crystal would exhibit a marked dependence on crystal direction, and we set about at once preparing experiments for an investigation of this dependence. We must admit that the results obtained in these experiments have proved to be quite at variance with our expectations. It seemed likely that strong beams would be found issuing from the crystal along what may be termed its transparent directions -- the directions in which the atoms in the lattice are arranged along the smallest number of lines per unit area. Strong beams are indeed found issuing from the crystal, but only when the speed of bombardment lies near one or another of a series of critical values, ant then in directions quite unrelated to crystal transparency.
"The most striking characteristic of these beams is a one to one correspondence ...which the strongest of them bear to the Laue beams that would be found issuing from the same crystal if the incident beam were a beam of x-rays. Certain others appear to be analogues ... of optical diffraction beams from plane reflection gratings -- the lines of these gratings being lines or rows of atoms in the surface of the crystal. Because of these similarities ... a description ... in terms of an equivalent wave radiation ... is not only possible, but most simple and natural. This involves the association of a wavelength with the incident electron beam, and this wavelength turns out to be in acceptable agreement with the value h/mv of the undulatory mechanics, Planck's action constant divided by the momentum of the electron.
"That evidence for the wave nature of particle mechanics would be found in the reaction between a beam of electrons and a single crystal was predicted by Elsasser two years ago -- shortly after the appearance of L. de Broglie's original papers on wave mechanics."
The above quotes are from Physical Review 30, 705 (1927).
Notes Copyright Ó 1997 Michael Fowler