- a.
- Give a qualitative answer.
- b.
- Suppose you are burning wood at the rate of 5 kg per hour. At
what rate are you producing energy (in watts) if the burning wood releases
3500 kcalories per kg?
- c.
- How much hot air goes up the chimney (in cubic meters per second)
if the air is heated by 200 kelvin? In an open fireplace, most heat goes up
the chimney.
- a.
- Give a qualitative answer.
- b.
- Determine the heat diffusion constant in air, D, from
dimensional analysis. Argue that D can depend on the speed of air
molecules, v, and on the mean free path between collisions, l, but not
on the mass of the molecules, m, (except that m affects v). This is
enough to find D, up to a constant which we take to be 1, but is actually
more like 1/3. (If you worry D can depend also on the mean time between
collisions,
 , note that v = l/tau, up to a factor of order 1.)
, note that v = l/tau, up to a factor of order 1.)
- c.
- What is v? As you recall, the mean kinetic energy per molecule
at temperature
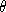 is (3/2)k<sub>B theta. From this, find v
for the N
is (3/2)k<sub>B theta. From this, find v
for the N 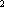 and O
and O 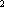 molecules. Which molecule moves faster? What is
the typical value of v at room temperature (averaged over molecular
species, if you wish)?
molecules. Which molecule moves faster? What is
the typical value of v at room temperature (averaged over molecular
species, if you wish)?
- d.
- Next, what is l? As we discussed in class, it obeys the
relation
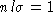 , where n is the number density of molecules and
, where n is the number density of molecules and  is the collision cross-section, about equal to
is the collision cross-section, about equal to 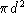 , with
d the diameter of a molecule. Give numbers for n and l at room
temperature, assuming
, with
d the diameter of a molecule. Give numbers for n and l at room
temperature, assuming  m.
m.
- e.
- You can now obtain a formula for D in terms of
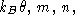 and d. Check the dimensions. Compute the numerical value of D
at room temperature and pressure and check with the value in Table 6.6 of
the PQRG. Is it much different at the temperature of frozen food?
and d. Check the dimensions. Compute the numerical value of D
at room temperature and pressure and check with the value in Table 6.6 of
the PQRG. Is it much different at the temperature of frozen food?
- f.
- Knowing D obtain a formula for the thermal conductivity of
air. Check with the numerical value in the PQRG.
- g.
- Finally, compute the R value of a 10 cm layer of undisturbed
cold air near the top of an open frozen food bin.
- a.
- If
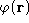 is a scalar function, show that
is a scalar function, show that 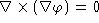 .
.
- b.
- If
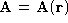 is a vector function, show that
is a vector function, show that 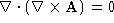 .
.
- c.
- Suppose that in two dimensions
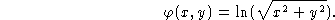
Calculate
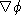 . Express your result in polar coordinates
. Express your result in polar coordinates
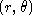 .
.
- d.
- Plot the vector field which you calculated in (c).