- ...pressure
![[*]](http://www.phys.virginia.edu/usr/icons.gif/foot_motif.gif)
- In general, the specific heat is dQ/dT, or TdS/dT. This can be rewritten
using the basic equation of thermodynamics (just energy balance), dE = TdS - pdV.
At constant volume, dE = TdS and
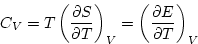
At constant pressure, it is convenient to work with the quantity H = E + pV, called the enthalpy.
We find dH = dE + pdV + Vdp = TdS + Vdp, and so
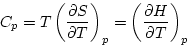
In an ideal gas, dE = CVdT and pV = NkBT, so that dH = dE + d(pV) = (CV + NkB)dT and Cp = CV + NkB.
V. Celli, Univ. of Virginia
11/10/1997
![]()
![]()
![]()
![]()