


Next: About this document ...
Phys 312 - Assignment 10 - Due 9 Apr 98
- 1.
- Radiation and scattering: why is the sky blue? As one
application of the Larmor radiation formula we will study the excitation of
an atom by an incident plane EM wave. The interaction of the electric field
of the incident plane wave with the electrons and the nucleus causes them to
oscillate at the same frequency as the wave, resulting in an oscillating
electric dipole (the magnetic forces are entirely negligible except for very
intense incident waves). The accelerations of the electrons are large
compared to the acceleration of the nucleus, due to the very large mass
ratio. The oscillating dipole then causes the atom to radiate electric
dipole radiation, as discussed in class.
- (a)
- Consider a simple model in which one electron is bound harmonically
to the nucleus (i.e., the electron is subject to a restoring force
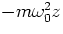 , with m the electron mass,
, with m the electron mass, 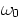 a natural atomic
frequency, and z the displacement from the origin). The electric field of
the incident plane wave at the atom has the form
a natural atomic
frequency, and z the displacement from the origin). The electric field of
the incident plane wave at the atom has the form 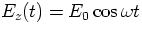 (we can manage well without complex notation in this problem). Assuming
that
(we can manage well without complex notation in this problem). Assuming
that 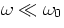 , show that the electron moves in phase with
the electric field with an acceleration
, show that the electron moves in phase with
the electric field with an acceleration
| 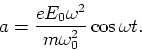 |
(1) |
- (b)
- Use the Larmor radiation formula to show that the time-averaged power
radiated by the charge is
| 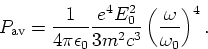 |
(2) |
Express this in terms of the wavelength 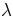 of the incident wave to
show that
of the incident wave to
show that 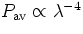 , which is a famous law derived
by Lord Rayleigh. This result shows that short wavelength radiation is
scattered more effectively by atoms than long wavelength radiation. This
result is valid for
, which is a famous law derived
by Lord Rayleigh. This result shows that short wavelength radiation is
scattered more effectively by atoms than long wavelength radiation. This
result is valid for 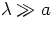 , with a the characteristic size of the
atom.
, with a the characteristic size of the
atom.
- (c)
- Use the above result to explain (i) why the sky is blue, (ii) why
sunsets are red, and (iii) why it is easier to get sunburned at midday.
- (d)
- Finally, think a bit about the polarization of the scattered
radiation. Suppose you take some Polaroid sunglasses and look northward as
the sun sets in the west. If you rotate the sunglasses you'll notice marked
intensity variations (try it). Why? Please be as specific as possible.
Figure 4.3(c) in Melissinos may help. See also the lecture
notes.
- 2.
- The plasma frequency and the ionosphere.
- (a)
- Note that eq. 4.34 in Melissinos becomes equivalent to eq. 4.42
in the appropriate limit. What is this limit and what does it mean,
physically?
- (b)
- The plasma frequency for the ionosphere is given on page 135 by Melissinos. Electrons in a metal behave much like those in the ionosphere,
but their density is much higher. Estimate the plasma frequency of copper
and explain why many metals become transparent in the ultraviolet.
- 3.
- Physics vocabulary. The following terms (which are used by Melissinos) have specific meanings in the physical sciences, although they
may seem equivalent or vague to the uninitiated. Give a short definition and
an example of each.
- (a)
- Diffusion
- (b)
- Dispersion
- (c)
- Diffraction
- (d)
- Refraction
- (e)
- Birefringence
- (f)
- Scattering
- 4.
- Lifetime of the sun. The flux of radiant energy from the sun at
the earth's surface is approximately 1.4 kW/m2.
- (a)
- From this estimate the total power produced by nuclear reactions in
the sun; from your knowledge of the proton-proton cycle, estimate the rate
of hydrogen burning in the sun; finally, estimate the amount of time before
the sun ``burns out.''
- (b)
- Now assume that the sun's energy comes from chemical reactions.
Estimate the lifetime of this ``chemical'' sun. [Note: exactly this
calculation was carried out by Lord Kelvin at the end of the 19th century.
His estimate was substantially larger than ``biblical'' estimates, but much
smaller than geological estimates based on sedimentation rates.]



Next: About this document ...
Vittorio Celli
4/6/1998
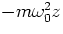 , with m the electron mass,
, with m the electron mass, 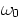 a natural atomic
frequency, and z the displacement from the origin). The electric field of
the incident plane wave at the atom has the form
a natural atomic
frequency, and z the displacement from the origin). The electric field of
the incident plane wave at the atom has the form 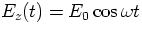 (we can manage well without complex notation in this problem). Assuming
that
(we can manage well without complex notation in this problem). Assuming
that 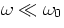 , show that the electron moves in phase with
the electric field with an acceleration
, show that the electron moves in phase with
the electric field with an acceleration
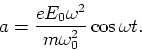
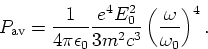
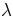 of the incident wave to
show that
of the incident wave to
show that 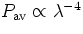 , which is a famous law derived
by Lord Rayleigh. This result shows that short wavelength radiation is
scattered more effectively by atoms than long wavelength radiation. This
result is valid for
, which is a famous law derived
by Lord Rayleigh. This result shows that short wavelength radiation is
scattered more effectively by atoms than long wavelength radiation. This
result is valid for 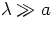 , with a the characteristic size of the
atom.
, with a the characteristic size of the
atom.