Adapted from
Eric's Treasure Trove.
Links shown in blue take you back to the original site.
 Danish physicist who proposed a successful quantum model of the atom in 1913. His model assumed that
(1) the electron exists on precise circular orbits around the nucleus,
(2) as long as an electron remains in one orbit, no energy is given off, and
(3) the angular momenta associated with allowed electron motion are integral multiples of
Danish physicist who proposed a successful quantum model of the atom in 1913. His model assumed that
(1) the electron exists on precise circular orbits around the nucleus,
(2) as long as an electron remains in one orbit, no energy is given off, and
(3) the angular momenta associated with allowed electron motion are integral multiples of 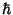 .
Bohr introduced the Correspondence Principle, which states that quantum mechanical formulas must reduce to the
classical results in the limit of large quantum number. He also advocated a probabilistic interpretation of
quantum mechanics known as the
Copenhagen Interpretation.
.
Bohr introduced the Correspondence Principle, which states that quantum mechanical formulas must reduce to the
classical results in the limit of large quantum number. He also advocated a probabilistic interpretation of
quantum mechanics known as the
Copenhagen Interpretation.
In 1922 he was awarded the
Nobel Prize in physics for this work.
Additional biographies:
MacTutor (St. Andrews) and the
Nobel site.
References
Blaedel, N. Harmony and Unity: The Life of Niels Bohr. Madison, WI: Science Tech, 1988.
Murdoch, D. Niels Bohr's Philosophy of Physics. New York: Cambridge University Press, 1987.
Pais, A. Niels Bohr's Times: In Physics, Philosophy, and Polity. Oxford, England: Oxford University Press, 1991.
Petruccioli, S. Atoms, Metaphors, and Paradoxes: Neils Bohr and the Construction of a New Physics. Cambridge University Press, 1994.
Rozenthal, S. (Ed.). Niels Bohr: His Life and Work as Seen by His Friends and Colleagues.
New York: Elsevier, 1985.
Whitaker, A. Einstein, Bohr, and the Quantum Dilemma. 1995.
© 1996-9 Eric W. Weisstein
1999-01-22
 Danish physicist who proposed a successful quantum model of the atom in 1913. His model assumed that
(1) the electron exists on precise circular orbits around the nucleus,
(2) as long as an electron remains in one orbit, no energy is given off, and
(3) the angular momenta associated with allowed electron motion are integral multiples of
Danish physicist who proposed a successful quantum model of the atom in 1913. His model assumed that
(1) the electron exists on precise circular orbits around the nucleus,
(2) as long as an electron remains in one orbit, no energy is given off, and
(3) the angular momenta associated with allowed electron motion are integral multiples of