Our Understanding of Matter
Atomic View of Matter
All matter is made of units called atoms. For practical purposes, an atom may
be visualized as a minature version of our solar system. In the center is
something called the nucleus, which accounts for almost all of the mass of the
atom, and is positively charged. Orbiting around the nucleus are one or more
electrons, which are small, point-like particles whose negative charge attracts
them to the nucleus, thus providing the necessary force to create their orbit.
So far, our experiments tell us that the electron is not made up of any
constituent parts, i.e., it is a fundamental particle that cannot be further
split or divided. The nucleus, on the other hand, has been known for some time
now to be made up of two types of smaller particles. These two types of
particles are the proton and the neutron.
Sub-atomic View of Matter
In many respects the proton and neutron are similar: they have nearly the same
mass, the same spin, they can bind with other particles in similar ways, etc.
The main difference between the two, however, is that the proton is positively
charged, whereas the neutron has no charge. In the next section we will see
that this is because the proton has different building blocks than the neutron.
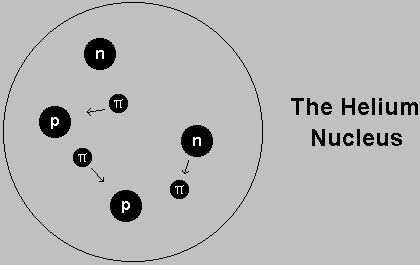
A given nucleus may consist of many protons and many neutrons, usually in
roughly equal quantities. The protons and neutrons are able to form a nucleus
by exchanging a particle, called a pion, that helps to convey a force between
them. It is the force derived from pion exchange that overcomes the repulsive
force between the protons due to their similar charge (like charges repel).
Because this pion-derived force is strong enough to overcome the electrostatic
repulsion between protons, the name Strong Force has been given to it. We
will see that this is just a special case of the Strong Force, the more general
case being the force that binds quarks together.
Quark Model of Matter
In the 60's and 70's scientists created theories, and then experiments, to show
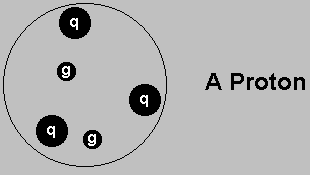
that protons and neutrons are also made up of even smaller particles. These
particles are called Quarks, and there are three of them in every proton, and
three of them in every neutron. Quarks also have electric charge (both
positively and negatively charged quarks exist), and a similar phenomenon binds
them together as what occured in nucles binding in the previous section: the
natural repulsion due to similar electric charge of the quarks is overcome by
the force derived by the quarks exchanging a particle, this time the particle
is called a gluon.
It is our current understanding that quarks are not made up of even smaller
particles, and therefore may, like the electron, be considered fundamental
particles.
Current Research
Although much work is still being done to investigate the manner in which the
Strong Force binds protons and neutrons together in a nucleus, a new field has
emerged with the discovery of the quark. Since it is again the Strong Force
binding quarks together into a proton (or neutron), experiments can now be
constructed to study the Strong Force by looking at quark binding.
Summary
All matter is made of molecules. Molecules are the smallest unit of matter to
exhibit distinct chemical properties.

All molecules are made of atoms. These are what show up on the Periodic Table
of the Elements.
The nucleus of all atoms is made of protons and neutrons, held together by
their exchange of pions.
Protons are made of three quarks, bound together by their exchange of gluons.
The same goes for neutrons.
Back to the Neutron Detector home page
Donal Day, University of Virginia