For a number of years we have been using ellipsometry to study physisorbed films on graphite (HOPG), mostly in the thickness range of one to eight or ten layers [1,2,3]. Compared to the more common volumetric technique, this method is rapid and requires only a small substrate area; consequently the pressure resolution is superior and capillary condensation is avoided for thick films.
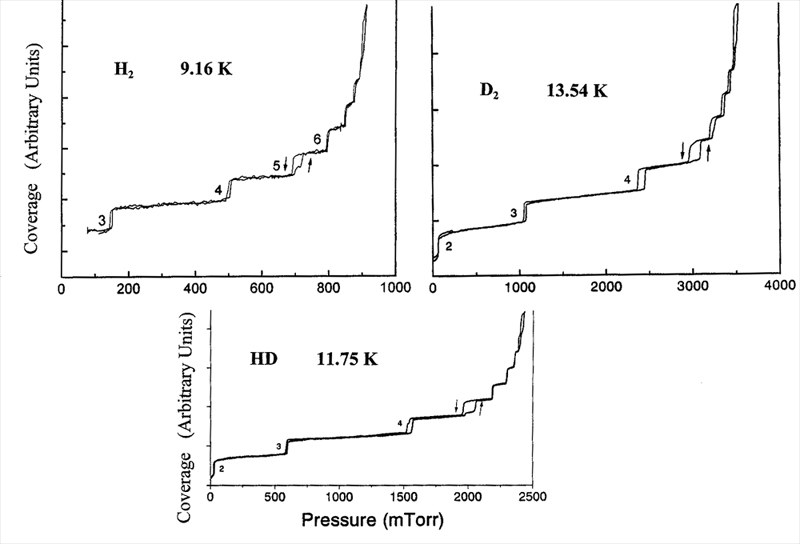
Hong Wu applied this method to detailed surveys of adsorption of neon [4], H2, HD [5], D2, and CO on graphite. For example, Figure 1 shows coverage isotherms for the three hydrogen isotopomeres at temperatures selected to display a characteristic hysteresis in the fourth and fifth layer adsorption-desorption steps. This evidently reflects a structural change in the films at this thickness, which has not been seen by other techniques.

David Boyd constructed a cryostat which allows measuring infrared reflection-absorption spectra (variously designated IRAS, IRRAS, or RAIS) concurrently with fixed-wavelength ellipsometry, on opposite sides of the same graphite slab. IRRAS is used routinely in the study of chemisorption on metal surfaces and the groups of Ewing (Indiana) and Heidberg (Hannover) have made extensive use of transmission IR spectroscopy to study physisorption systems such as CO and CO2 on cleaved surfaces of NaCl and MgO. Past applications of IRRAS to adsorption on graphite are few: In 1990 Heidberg et al. [A] reported spectra for CO on graphite and more recently Nalezinski, Bradshaw, and Knorr [B,C] have studied adsorption of CF2Cl2 and CH3Cl. For suitable molecules, this technique gives information on the molecular orientation and/or the coverage, and on interactions between the adsorbate molecules and with the substrate. Graphite is a sufficiently good conductor in the infrared that the surface selection rule applies, as for metals (i.e., only modes with a dipole component perpendicular to the surface can be observed).
So far we have studied CO, CD4, C2D6, SF6 [6], CF4, CF3Cl, and C2F6 [7]. For the C2D6 monolayer we see modes with polarization along and perpendicular to the molecular axis. For the tetrahedral and octahedral molecules the observed modes are three-fold degenerate, so coverage and interaction but no orientational information can be obtained. The strong absorbers CF4 and SF6 provide an opportunity to test dynamic dipole interaction theory for multiple layers as well as for monolayers. The Figure 2 below shows the absorption frequency measured for CF4 monolayer to four-layer films as a function of vapor pressure for various temperatures. The abscissa is the chemical potential difference "mu-mu2" = T ln(p/p2), where p2 is the pressure at which the second layer of CF4 condenses at that temperature.

Although it is becoming clear that IRRAS is a valuable technique for studying individual adsorbates on graphite, our primary objective is a better technique for studying binary mixture adsorbates. A great deal of work has been done on such systems in recent years, but often the scope of individual studies has been limited due to the extra thermodynamic parameter and the availability of only a single probe in most cases. A common observation is displacement of a pre-adsorbed layer by a less condensible second component. In a volumetric experiment, what is measured is the amount of the second component adsorbed and the interpretation sometimes has been difficult or ambiguous. In a few cases a supplementary probe such as x-ray or neutron diffraction has provided additional information on one or both components. IRRAS can, in suitable cases, provide essential information on the state of the pre-adsorbed layer and can be used concurrently with a basic coverage measurement.
To demonstrate this we have used ellipsometry and IRRAS together to study adsorption of krypton, methane, or CF4 on graphite pre-coated with a monolayer of SF6. We have studied also SF6 - Xe mixed monolayers at and below the saturation chemical potential of SF6. For this system we find an upper consolute critical point, above which there is complete solubility and the SF6 monolayer is displaced by continuous dilution as the chemical potential of CF4 is increased. Currently we are studying CF4 + C2F6.