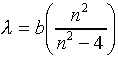
Michael Fowler University of Virginia
Physics 252 Home Page
Link to Previous Lecture
The first person to realize that white light was made up of the colors
of the rainbow was Isaac Newton, who in 1666 passed sunlight through a
narrow slit, then a prism, to project the colored spectrum on to a wall.
This effect had been noticed previously, of course, not least in the sky,
but previous attempts to explain it, by Descartes and others, had suggested
that the white light became colored when it was refracted, the color depending
on the angle of refraction. Newton clarified the situation by using a second
prism to reconstitute the white light, making much more plausible the idea
that the white light was composed of the separate colors. He then took
a monochromatic component from the spectrum generated by one prism and
passed it through a second prism, establishing that no further colors were
generated. That is, light of a single color did not change color on refraction.
He concluded that white light was made up of all the colors of the rainbow,
and that on passing through a prism, these different colors were refracted
through slightly different angles, thus separating them into the observed
spectrum.
In 1752, the Scottish physicist Thomas Melvill discovered that putting
different substances in flames, and passing the light through a prism,
gave differently patterned spectra. Ordinary table salt, for example, generated
a "bright yellow". Furthermore, not all the colors of the rainbow
appeared - there were dark gaps in the spectrum, in fact for some materials
there were just a few patches of light. By the 1820's, Herschel had recognized
that spectra provided an excellent way to detect and identify small quantities
of an element in a powder put into a flame.
Meanwhile, the white light of the sun was coming in for more detailed scrutiny. In 1802, William Wollaston in England had discovered (perhaps by using a thinner slit or a better prism) that in fact the solar spectrum itself had tiny gaps - there were many thin dark lines in the rainbow of colors. These were investigated much more systematically by Joseph von Fraunhofer, beginning in 1814. He increased the dispersion by using more than one prism. He found an "almost countless number" of lines. He labeled the strongest dark lines A, B, C, D, etc.
In 1849, Foucault (of speed of light and pendulum fame) examined the spectrum of light from a voltaic arc between carbon poles. He saw a bright double yellow line at exactly the same wavelength as Fraunhofer's dark D line in the solar spectrum. Investigating further, Foucault passed the sun's light through the arc, then through a prism. He observed that the D lines in the spectrum were even darker than usual. After testing with other sources, he concluded that the arc, which emitted light at the D line frequency, would also absorb light from another source at that frequency.
This discovery did not surprise Sir George Stokes in Cambridge. He pointed
out that any mechanical system with a natural frequency of oscillation
will emit at that frequency if disturbed, but will also absorb most readily
at that frequency from incoming disturbances, the phenomenon of resonance
(Dampier page 241).
Question: In a total eclipse of the sun, the only sunlight reaching earth comes from the hot gases of the sun's atmosphere, light from the sun's main disc being blocked by our moon. The light from these hot gases was analyzed during an eclipse in 1870. How do you think the spectrum observed related to that of full sunlight?
The spectrum of hydrogen, which turned out to be crucial in providing the first insight into atomic structure over half a century later, was first observed by Anders Angstrom in Uppsala, Sweden, in 1853. His communication was translated into English in 1855. Angstrom, the son of a country minister, was a reserved person, not interested in the social life that centered around the court. Consequently, it was many years before his achievements were recognized, at home or abroad (most of his results were published in Swedish). Meanwhile, in Freeport, Pennsylvania, in 1855, David Alter described the spectrum of hydrogen and other gases. In the 1840's, Alter had started the first commercial production of bromine from brines. He also found a way to extract oil from coal, but that proved uneconomic after the discovery of oil in Pennsylvania. His work was not widely recognized, either. (Dampier, BDS)
The first really systematic investigation of spectra was that of Bunsen
and Kirchhoff, in Heidelberg, between 1855 and 1863. They used several
techniques. For one thing, they introduced various salts into -- what else?
-- the flame of a Bunsen burner. This was a very effective way of viewing
spectra, because the Bunsen burner flame itself gave out practically no
light. They also used the cooler flame of alcohol burning mixed with water
to generate a vapor to study absorption spectra. Finally, they studied
the spectra of electric arcs between electrodes of different materials.
Using iron electrodes gave a spectrum that coincided with dark lines in
the sun's spectrum. Copper electrodes did not. They concluded that the
sun's atmosphere contained iron, but not much copper, and that, they said,
seemed very plausible since there is so much iron in the earth, and in
meteors.
(Cautionary note to philosophers: In 1835, the French philosopher
Auguste Comte (the founder of positivism) wrote: "
Our knowledge concerning
the gaseous envelopes [of stars] is necessarily limited to their existence,
size
and refractive power, we shall not at all be able to determine their
chemical composition or even their density
I regard any notion concerning
the true mean temperature of the various stars as forever denied to us."
(Pais, IB, page 155))
The collaboration of Kirchhoff and Bunsen was a major research effort,
even by modern standards. They determined thousands of spectral lines,
each to an accuracy of one part in ten thousand. They spectroscopically
discovered new elements: rubidium and cesium. Their method was used to
find fifteen more new elements before the end of the century. In 1869,
Joseph Lockyer studied the spectra of solar prominences (in eclipses).
He found the spectra to be slightly Doppler shifted, so was able to deduce
the speeds of the gases whirling around the sunspots. He also found a spectrum
never seen before, and conjectured that it came from a new element he named
Helium.
In fact, helium was later discovered on earth in 1895, by Ramsay. At that time, it had just become evident that there was an inert component, argon, in the earth's atmosphere. Earlier, an inert gas had been observed to emanate from uranium salts when they were heated. Ramsay assumed this would be the same gas, but decided to check. On heating uranium salts and performing a spectral analysis of the emitted gas, much to his surprise he found it to be helium. (Yet another example of the Scientific Method at work: viz., almost all important discoveries are made accidentally while looking for something else.)
It is clear from the above that a tremendous amount of scientific progress
was made using spectral lines, yet no-one had the slightest idea why atoms
emitted at the frequencies they did. It was appreciated that spectra implied
that atoms had structure. In 1852, Stokes had stated that probably the
vibrations that produced light were vibrations among the constituent parts
of molecules (a term which also included atoms at that time) and in 1875
Maxwell, in enumerating properties atoms must have, included the capability
of internal motion or vibration. This worried Maxwell, though. As he said,
the spectroscopic evidence forces the conclusion that the atom is quite
complex, with many internal degrees of freedom. Yet apparently all these
modes of vibration, or almost all of them, are not excited by heat, since
if they were this extra capacity of the atom to absorb energy would be
reflected in its specific heat (Pais, IB page 175).
Obviously, if any pattern could be discerned in the spectral lines for an atom, that might be a clue as to the internal structure of the atom. One might be able to build a model. A great deal of effort went into analyzing the spectral data from the 1860's on. The big breakthrough was made by Johann Balmer, a math and Latin teacher at a girls' school in Basel, Switzerland. Balmer had done no physics before, and made his great discovery when he was almost sixty. He decided that the most likely atom to show simple spectral patterns was the lightest atom, hydrogen. Angstrom had measured the four visible spectral lines to have wavelengths 6562.10, 4860.74, 4340.1 and 4101.2 in Angstrom units (10-10 meters). Balmer concentrated on just these four numbers, and found they were given by the formula:
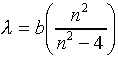
where b = 3645.6 Angstroms, and n = 3, 4, 5, 6. Balmer
suggested that there would be other lines - in the infrared - corresponding
to n = 7, 8, etc., and in fact some of them had already been observed,
unbeknownst to Balmer. He further conjectured that the 4 could be replaced
by 9, 16, 25,
and this also turned out to be true - but these lines,
further into the infrared, were not detected until the early twentieth
century, along with the ultraviolet lines generated by replacing the 4
by 1.
It is instructive to write Balmer's general formula in terms of the
inverse wavelength. This is called the wave number - the number of waves
that fit in one unit of length.
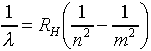
where n, m are integers, and RH is the
Rydberg constant, 109,737 cm-1.
This constant is named after the Swedish physicist Rydberg who
(in 1888) presented a generalization of Balmer's formula, in which the integer
n was replaced by n + constant, the constant being less than
unity. Rydberg suggested that all atomic spectra formed families with this
pattern. (He also said he was unaware of Balmer's work.) It turns out that
there are families of spectra following Rydberg's pattern, notably in the
alkali metals, sodium, potassium, etc., but not with
the precision the hydrogen atom lines fit the Balmer formula, and low values
of n give lines that deviate considerably.
(Modern footnote: atoms having spectral lines following Rydberg's formula are called Rydberg atoms. As we shall see later, these Rydberg atoms have one electron orbiting at a much greater distance from the nucleus than the others. Consequently, Rydberg atoms can only survive in a gas at very low pressure, otherwise that outermost electron gets knocked off. Prof. Tom Gallagher in our Department is a world expert on these atoms, which have proven a rich source of information on the quantum mechanics of atomic structure.)
One pattern that was noticed in the spectra of many atoms is Ritz'
Combination Principle:
if for a given atom there are spectral lines at two wave numbers, there
is sometimes another spectral line at the precise sum of those two wave numbers.
It is easy
to see from Balmer's formula that this is true for some pairs of lines in the hydrogen spectrum. It also
turns out to be true for atoms where the spectral lines have no other discernible pattern.
Books I used in preparing this lecture:
Inward Bound, Abraham Pais, Oxford, 1986
Collins' Biographical Dictionary of Scientists, HarperCollins,
Glasgow, 1994
A History of Science, W. C. Dampier, Cambridge, 1929.
Physics 252 Home Page
Link to Next Lecture
Copyright ©1997 Michael Fowler