Michael Fowler
University of Virginia
Physics 252 Home Page
Link to Previous Lecture
The first attempt to construct a physical model of an atom was
made by William Thomson (later elevated to Lord Kelvin) in 1867.
The most striking property of the atom was its permanence.
It was difficult to imagine any small solid entity that could
not be broken, given the right force, temperature or chemical
reaction. In contemplating what kinds of physical systems exhibited
permanence, Thomson was inspired by a paper Helmholtz had written
in 1858 on vortices. This work had been translated into
English by a Scotsman, Peter Tait, who showed Thomson some ingenious
experiments with smoke rings to illustrate Helmholtz' ideas.
The main point was that in an ideal fluid, a vortex line
is always composed of the same particles, it remains unbroken,
so it is ring-like. Vortices can also form interesting combinations
-- A good demonstration is provided by creating two vortex rings
one right after the other going in the same direction. They can
trap each other, each going through the other in succession. This
is probably what Tait showed Thomson, and it gave Thomson the
idea that atoms might somehow be vortices in the ether.
Of course, in a non ideal fluid like air, the vortices dissipate
after a while, so Helholtz' mathematical theorem about their permanence
is only approximate. But Thomson was excited because the ether
was thought an ideal fluid, so vortices in the ether might
last forever! This was very aesthetically appealing to everybody
- "Kirchhoff, a man of cold temperament, can be roused to
enthusiasm when speaking of it." (Pais, IB page 177, source
for this material). In fact, the investigations of vortices,
trying to match their properties with those of atoms, led to a
much better understanding of the hydrodynamics of vortices - the
constancy of the circulation around a vortex, for example, is
known as Kelvin's law. In 1882 another Thomson, J. J., won a
prize for an essay on vortex atoms, and how they might interact
chemically. After that, though, interest began to wane - Kelvin
himself began to doubt that his model really had much to do with
atoms, and when the electron was discovered by J. J. in 1897,
and was clearly a component of all atoms, different kinds of non-vortex
atomic models evolved.
It is fascinating to note that the most exciting theory of fundamental particles at the present time, string theory, has a definite resemblance to Thomson's vortex atoms. One of the basic entities is the closed string, a little loop, which has fields flowing around it reminiscent of the swirl of ethereal fluid in Thomson's atom. And it's a very beautiful theory - Kirchhoff would have been enthusiastic!
In 1878, Alfred Mayer, at the University of Maryland, dreamed
up a neat demonstration of how he imagined atoms might be arranged
in molecules. He took a few equally magnetized needles and stuck
them through corks so that they would float with their north poles
all at the same height above the water, all repelling each other
equally. He then held the south pole of a more powerful magnet
some distance above the water, to attract the needles towards
this central point. The idea was to see what equilibrium patterns
the needles would form for different numbers of needles. He found
something remarkable - the needles liked to arrange themselves
in shells. Three to five magnets just formed a triangle, square
and pentagon in succession. but for six magnets, one went to the
center and the others formed a pentagon. For more magnets, an
outer shell began to form.
Kelvin's immediate response to Mayer's publication was that this should give some clues about the vortex atom. Apparently it didn't, but twenty-five years later it guided his thinking on a new model.
Kelvin, in 1903, proposed that the atom have the newly discovered
electrons embedded somehow in a sphere of uniform positive charge,
this sphere being the full size of the atom. (Of course, the sphere
itself must be held together by unknown non-electrical forces
- which is still true of the positive charge in our modern model
of the atom.) This picture was taken up by J. J. Thomson too,
and was dubbed the plum pudding model, after traditional English
Christmas fare, a large round pudding (rich with suet) with raisins
embedded in it. In 1906, J. J. concluded from an analysis of the
scattering of X-rays by gases and of absorption of beta-rays by
solids, both of which he assumed were effected by electrons, that
the number of electrons in an atom was approximately equal to
the atomic number. This led to a picture of electron arrangements
in an atom reminiscent of Mayer's magnets. Perhaps by analyzing
possible modes of vibration of electrons in these configurations,
the spectra could be calculated.
The simplest case to consider was clearly hydrogen, now assumed (correctly) to contain just one electron.
By "color" we mean here the spectral colors emitted
when the atom is excited. In Thomson's plum pudding model, there
is a clear relationship between the size of the pudding
and the frequency at which the electron will oscillate,
and hence presumably radiate, when excited. The two are related
because the assumption is that the total positive charge - which
is uniformly spread throughout the sphere -- is just equal to
the electron's negative charge. At rest in its lowest state, the
electron just sits in the middle of this sphere of charge. When
bumped somehow, it will oscillate about that point. If the electron
is at distance x from the center, it will feel a restoring
force towards the center equal to the attraction from that part
of the positive charge it is "outside" of - that is,
the charge within a sphere of radius x about the center.
Therefore, the larger the whole atom -- the pudding -
the more thinly spread the positive charge is, and the smaller
the amount of charge within the small sphere of radius x
that is attracting the electron back towards the center. So,
the bigger the atom is, the slower the electron's oscillation
is, and the lower frequency the radiation emitted.
It is straightforward to give a quantitative estimate of the size
of the atom based on the observation that when excited it emits
radiation in the visible range.
Let us assume that the positively charged sphere has radius r0
(this is then the size of the atom, which we know is about 10-10
meters).
If the electron is displaced from the center of the atom in the
x-direction an amount x, it is attracted back by
all the charge that is now closer to the center than itself, that
is, an amount of charge equal to ex3/r03.
(Recall e is the total amount of charge on the sphere,
and x3/r03 is the
fraction of the sphere closer to the center than x.) This
charge acts as if it were a point charge at the origin, so the
inverse-square law gives a 1/x2 factor, and
the equation of motion for the electron is therefore:
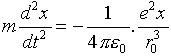
Provided it stays within the sphere, the electron will execute simple harmonic motion with a frequency
 .
.
Notice that, as we discussed above, as the size of the atom increases
the frequency goes down. And we know the frequency, at least approximately
--it corresponds to visible light. Therefore, this model will
predict a size of the atom, which we can compare with the size
from other predictions, such as Brownian motion (plus the assumption
that in a liquid, the atoms are fairly close packed - they take
up most of the room available).
If we take visible light, say with a frequency 4.1015
radians per second, we find r0 must be about
2.10-10 meters, a little on the large side, but encouragingly
close to the right answer for a first attempt.
Sad to report, though, no real progress was made beyond this in
predicting spectra using Thomson's pudding. Many attempts were
made to find stable arrangements of electrons in atoms, not just
hydrogen, using models like Mayer's magnets, and also having the
electrons going around in circles. It was hoped that if certain
numbers of magnets formed a very stable arrangement, that might
model a chemically nonreactive atom, etc. - but nobody succeeded
in making any real predictions along these lines, the models could
not be connected with the properties of real atoms.
Evidently, then, the theorists were stuck - and the experimental
challenge was to find some way to look inside an atom,
and see how the electrons were arranged. This is what Rutherford
did, as we shall discuss in the next lecture. He was very surprised
by what he saw.
Physics 252 Home Page
Link to Next Lecture
Copyright © 1997 Michael Fowler