Wave Equations
Michael Fowler
University of Virginia
Physics 252 Home Page
Link to Previous Lecture
Photons and Electrons
We have seen that electrons and photons behave in a very similar
fashion -- both exhibit diffraction effects, as in the double slit
experiment, both have particle like or quantum behavior. We can
in fact give a complete analysis of photon behavior -- we can figure
out how the electromagnetic wave propagates, using Maxwell's equations,
then find the probability that a photon is in a given small volume
of space dxdydz, |E|2dxdydz, the
energy density. On the other hand, our analysis of the electron's
behavior is incomplete -- we know that it must also be described
by a wave function 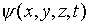 analogous to E, such
that
analogous to E, such
that 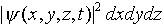 gives the probability of finding
the electron in a small volume dxdydz around the point
(x, y, z) at the time t. However,
we do not yet have the analog of Maxwell's equations to tell us
how
gives the probability of finding
the electron in a small volume dxdydz around the point
(x, y, z) at the time t. However,
we do not yet have the analog of Maxwell's equations to tell us
how 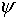 varies in time and space. The
purpose of this section is to give a plausible derivation of such
an equation by examining how the Maxwell wave equation works for
a single-particle (photon) wave, and constructing parallel equations
for particles which, unlike photons, have nonzero rest mass.
varies in time and space. The
purpose of this section is to give a plausible derivation of such
an equation by examining how the Maxwell wave equation works for
a single-particle (photon) wave, and constructing parallel equations
for particles which, unlike photons, have nonzero rest mass.
Maxwell's Wave Equation
Let us examine what Maxwell's equations tell us about the motion
of the simplest type of electromagnetic wave -- a monochromatic wave
in empty space, with no currents or charges present. First, we
briefly review the derivation of the wave equation from Maxwell's
equations in empty space:
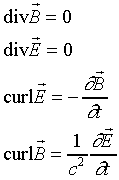
To derive the wave equation, we take the curl of the third equation:
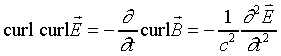
together with the vector operator identity
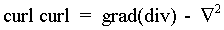
to give
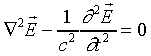 .
.
For a plane wave moving in the x-direction this
reduces to
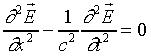
The monochromatic solution to this wave equation has the form
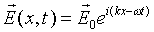 .
.
(Another possible solution is proportional to cos(kx - wt).
We shall find that the exponential form, although a complex number,
proves more convenient. The physical electric field can be taken
to be the real part of the exponential for the classical case.)
Applying the wave equation differential operator to our plane
wave solution
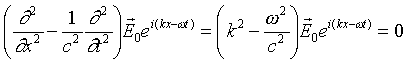 .
.
If the plane wave is a solution to the wave equation, this must
be true for all x and t, so we must have
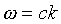 .
.
This is just the familiar statement that the wave must travel
at c.
What does the Wave Equation tell us about the Photon?
We know from the photoelectric effect and Compton scattering that
the photon energy and momentum are related to the frequency and
wavelength of the light by
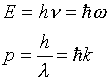
Notice, then, that the wave equation tells us that 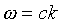 and hence E = cp.
and hence E = cp.
To put it another way, if we think of 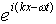 as
describing a particle (photon) it would be more natural to write
the plane wave as
as
describing a particle (photon) it would be more natural to write
the plane wave as
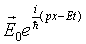
that is, in terms of the energy and momentum of the particle.
In these terms, applying the (Maxwell) wave equation operator
to the plane wave yields
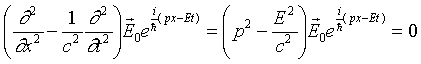
or
E2 = c2p2.
The wave equation operator applied to the plane wave describing
the particle propagation yields the energy-momentum relationship
for the particle.
Constructing a Wave Equation for a Particle with Mass
The discussion above suggests how we might extend the wave equation
operator from the photon case (zero rest mass) to a particle having
rest mass m0. We need a wave equation operator
that, when it operates on a plane wave, yields
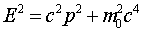
Writing the plane wave function
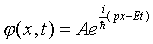
where A is a constant, we find we can get 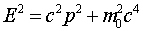 by adding a constant (mass) term to the differentiation terms
in the wave operator:
by adding a constant (mass) term to the differentiation terms
in the wave operator:
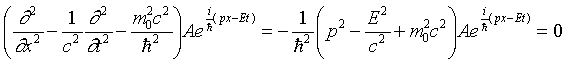 .
.
This wave equation is called the Klein-Gordon equation
and correctly describes the propagation of relativistic particles
of mass m0. However, it's a bit inconvenient
for nonrelativistic particles, like the electron in the hydrogen
atom, just as E2 = m02c4
+ c2p2 is less useful than
E= p2/2m for this case.
A Nonrelativistic Wave Equation
Continuing along the same lines, let us assume that a nonrelativistic
electron in free space (no potentials, so no forces) is described
by a plane wave:
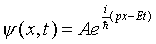 .
.
We need to construct a wave equation operator which, applied to
this wave function, just gives us the ordinary nonrelativistic
energy-momentum relationship, E = p2/2m.
The p2 obviously comes as usual from differentiating
twice with respect to x, but the only way we can get E
is by having a single differentiation with respect to time,
so this looks different from previous wave equations:
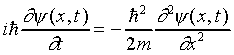 .
.
This is Schrödinger's equation for a free particle.
It is easy to check that if  has the plane
wave form given above, the condition for it to be a solution of
this wave equation is just E = p2/2m.
has the plane
wave form given above, the condition for it to be a solution of
this wave equation is just E = p2/2m.
Notice one remarkable feature of the above equation -- the i
on the left means that  cannot
be a real function.
cannot
be a real function.
How Does a Varying Potential Affect a de Broglie Wave?
The effect of a potential on a de Broglie wave was considered
by Sommerfeld in an attempt to generalize the rather restrictive
conditions in Bohr's model of the atom.
Since the electron was orbiting in an inverse square force, just
like the planets around the sun, Sommerfeld couldn't understand
why Bohr's atom had only circular orbits, no Kepler-like ellipses.
(Recall that all the observed spectral lines of hydrogen were
accounted for by energy differences between these circular orbits.)
De Broglie's analysis of the allowed circular orbits can be formulated
by assuming at some instant in time the spatial variation of the
wave function on going around the orbit includes a phase term
of the form 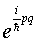 , where here the parameter
q measures distance around the orbit. Now for an acceptable
wave function, the total phase change on going around the orbit
must be 2n, where n is an integer. For the usual
Bohr circular orbit, p is constant on going around, q
changes by 2.pi.r, where r is the radius
of the orbit, giving
, where here the parameter
q measures distance around the orbit. Now for an acceptable
wave function, the total phase change on going around the orbit
must be 2n, where n is an integer. For the usual
Bohr circular orbit, p is constant on going around, q
changes by 2.pi.r, where r is the radius
of the orbit, giving
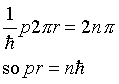
the usual angular momentum quantization.
What Sommerfeld did was to consider a general Kepler ellipse orbit,
and visualize the wave going around such an orbit. Assuming the
usual relationship 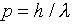 , the wavelength will
vary as the particle moves around the orbit, being shortest where
the particle moves fastest, at its closest approach to the nucleus.
Nevertheless, the phase change on moving a short distance delta_q
should still be
, the wavelength will
vary as the particle moves around the orbit, being shortest where
the particle moves fastest, at its closest approach to the nucleus.
Nevertheless, the phase change on moving a short distance delta_q
should still be 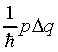 , and requiring the wave
function to link up smoothly on going once around the orbit gives
, and requiring the wave
function to link up smoothly on going once around the orbit gives
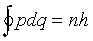
Thus only certain elliptical orbits are allowed. The mathematics
is nontrivial, but it turns out that every allowed elliptical
orbit has the same energy as one of the allowed circular orbits.
This is why Bohr's theory gave all the energy levels. Actually,
this whole analysis is old fashioned (it's called the "old
quantum theory") but we've gone over it to introduce the
idea of a wave with variable wavelength, changing with the
momentum as the particle moves through a varying potential.
Schrödinger's Equation for a Particle in a Potential
Let us consider first the one-dimensional situation of a particle
going in the x-direction subject to a "roller coaster"
potential. What do we expect the wave function to look like? We
would expect the wavelength to be shortest where the potential
is lowest, in the valleys, because that's where the particle is
going fastest -- maximum momentum. Perhaps slightly less obvious
is that the amplitude of the wave would be largest at the tops
of the hills (provided the particle has enough energy to get there)
because that's where the particle is moving slowest, and therefore
is most likely to be found.
With a nonzero potential present, the energy-momentum relationship
for the particle becomes the energy equation
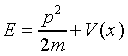 .
.
We need to construct a wave equation which leads naturally to
this relationship. In contrast to the free particle cases discussed
above, the relevant wave function here will no longer be a plane
wave, since the wavelength varies with the potential. However,
at a given x, the momentum is determined by the "local
wavelength", that is,
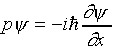 .
.
It follows that the appropriate wave equation is:
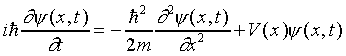 .
.
This is the standard one-dimensional Schrödinger equation.
In three dimensions, the argument is precisely analogous. The
only difference is that the square of the momentum is now a sum
of three squared components, for the x, y and z
directions, so
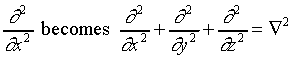 ,
,
and the equation is:
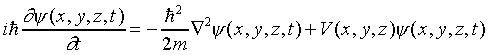 .
.
This is the complete Schrödinger equation.
Physics 252 Home Page
Link to Next Lecture
Copyright ©1997 Michael Fowler
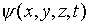 analogous to E, such
that
analogous to E, such
that 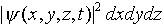 gives the probability of finding
the electron in a small volume dxdydz around the point
(x, y, z) at the time t. However,
we do not yet have the analog of Maxwell's equations to tell us
how
gives the probability of finding
the electron in a small volume dxdydz around the point
(x, y, z) at the time t. However,
we do not yet have the analog of Maxwell's equations to tell us
how 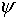 varies in time and space. The
purpose of this section is to give a plausible derivation of such
an equation by examining how the Maxwell wave equation works for
a single-particle (photon) wave, and constructing parallel equations
for particles which, unlike photons, have nonzero rest mass.
varies in time and space. The
purpose of this section is to give a plausible derivation of such
an equation by examining how the Maxwell wave equation works for
a single-particle (photon) wave, and constructing parallel equations
for particles which, unlike photons, have nonzero rest mass.
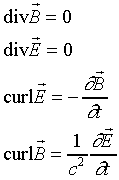
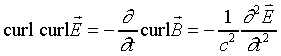
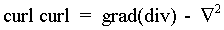
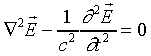 .
.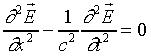
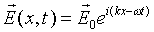 .
.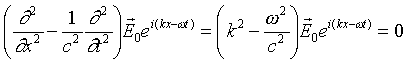 .
.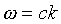 .
.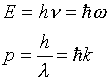
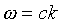 and hence E = cp.
and hence E = cp. 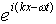 as
describing a particle (photon) it would be more natural to write
the plane wave as
as
describing a particle (photon) it would be more natural to write
the plane wave as
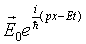
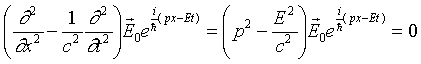
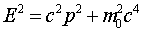
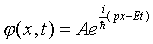
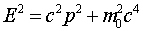 by adding a constant (mass) term to the differentiation terms
in the wave operator:
by adding a constant (mass) term to the differentiation terms
in the wave operator: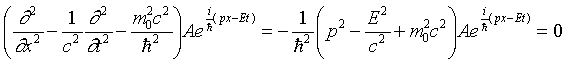 .
.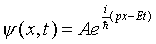 .
.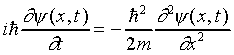 .
.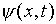 has the plane
wave form given above, the condition for it to be a solution of
this wave equation is just E = p2/2m.
has the plane
wave form given above, the condition for it to be a solution of
this wave equation is just E = p2/2m.
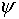 cannot
be a real function.
cannot
be a real function.
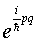 , where here the parameter
q measures distance around the orbit. Now for an acceptable
wave function, the total phase change on going around the orbit
must be 2n, where n is an integer. For the usual
Bohr circular orbit, p is constant on going around, q
changes by 2.pi.r, where r is the radius
of the orbit, giving
, where here the parameter
q measures distance around the orbit. Now for an acceptable
wave function, the total phase change on going around the orbit
must be 2n, where n is an integer. For the usual
Bohr circular orbit, p is constant on going around, q
changes by 2.pi.r, where r is the radius
of the orbit, giving
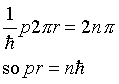
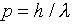 , the wavelength will
vary as the particle moves around the orbit, being shortest where
the particle moves fastest, at its closest approach to the nucleus.
Nevertheless, the phase change on moving a short distance delta_q
should still be
, the wavelength will
vary as the particle moves around the orbit, being shortest where
the particle moves fastest, at its closest approach to the nucleus.
Nevertheless, the phase change on moving a short distance delta_q
should still be 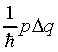 , and requiring the wave
function to link up smoothly on going once around the orbit gives
, and requiring the wave
function to link up smoothly on going once around the orbit gives
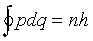
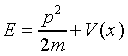 .
.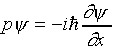 .
.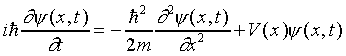 .
.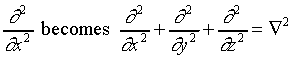 ,
, 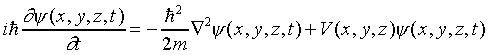 .
.