


Next: Phases of matter
Up: Fluid Mechanics: Preliminaries
Previous: Fluid Mechanics: Preliminaries
How does a fluid (liquid or gas) differ from a solid? We can answer this question either
in terms of microscopic properties or in terms of macroscopic properties.
- Solids:
- often have microscopic long-range order; the atoms or
molecules form a regular lattice (rubber and plastic are notable
exceptions);
- tend to form faceted crystals if grown under the right conditions;
- hurt when you kick them; they have a non-zero "shear modulus".
- Liquids:
- have microscopic short-range order, but no long-range order;
- flow under the influence of gravity;
- have zero shear modulus, so they flow aside when you kick them
(not too hard);
- have a fixed volume at low pressure and are usually hard to compresss.
- Gases:
- have very little short-range order (ideal gases have none);
- have zero shear modulus and you can easily move through them;
- expand to occupy the available volume and are highly compressible.
The shear modulus is a material property
which tells us how a substance
responds to shear forces, which change the shape of the substance without
changing the volume. This is illustrated in Fig. 2.1.
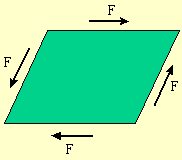
Figure 2.1: Shear forces acting on a fluid element.
A solid tries to resist static shear distortions, while a fluid does not. However, a sufficiently large stress will cause a solid to break (if
it is brittle), or to take a very different shape, perhaps permanently
(if it is ductile, like a hot steel rod). Conversely, some liquids are
highly viscous and change shape only slowly. Glasses are liquids
that increase their viscosity enormously as they cool, but can behave like
brittle solids under high stress. Obviously, rigid solids and freely flowing
liquids are just extreme cases.
The behavior under time-varying stress is more subtle, and inertia plays a
role. For instance, a nearly incompressible and non-viscous liquid, like
water, will not part under impact if it has no time to flow aside (as anyone
who has done a belly-flop can attest).
V. Celli, UVa
Mon Aug 11 22:46:35 EDT 1997
