Problem session 2
Solutions to problem set 1. Given hints on solving set 2 and getting pretty plots with MAPLE. See example at http://erwin.phys.virginia.edu/~jsf9k/hints.html. See also MAPLE short course notes, on reserve.
Lecture 4, Feb 2
Demos: shown "insides" of electrostatic air cleaner and laser printer.
Uniqueness of solution of Laplace's equation,
![]() , with assigned vaues
of V on the boundary (in practice, V has assigned values on various conductors).
How to proceed if the charges on the conductors are given, instead of the values of the potential.
Simplest example: Q = C(V1 - V2) for a capacitor with charges +Q and -Q
on the plates at potentials V1 and V2. C is the capacitance.
, with assigned vaues
of V on the boundary (in practice, V has assigned values on various conductors).
How to proceed if the charges on the conductors are given, instead of the values of the potential.
Simplest example: Q = C(V1 - V2) for a capacitor with charges +Q and -Q
on the plates at potentials V1 and V2. C is the capacitance.
Started review of quantum theory:
What is quantized and when? In periodic motion, action is quantized in units of h;
as a consequence, other quantities are quantized too, but not so simply.
What is action? It is a quantity with dimensions ML2T-1;
it can be energy × time, or momentum × length. Angular momentum has the same dimensions as action
and is simply quantized in units of 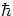 .
.
What is spin? It is something like the internal angular momentum of an elementary particle.
However, electrons (and quarks) have spin 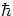 /2, while orbital angular momentum must be an
integer multiple of
/2, while orbital angular momentum must be an
integer multiple of 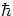 .
Photons have spin
.
Photons have spin 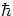 . One often says that electrons have spin 1/2 and photons
have spin 1.
. One often says that electrons have spin 1/2 and photons
have spin 1.
What is the Pauli exclusion principle? Read about it in Serway, page 860, or Tipler,
page 1206 and 1234, as it applies
to electrons. Actually, it applies to all elementary particles with spin 1/2 (called fermions) and is
mysteriously related to their unusual spin. It also applies to composite particles with half-integer spin
(1/2, 3/2, etc.).
References:
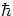 , so that some formulas look a little different. Physics
articles today almost always use
, so that some formulas look a little different. Physics
articles today almost always use 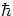 , unless they use "atomic units" in
which
, unless they use "atomic units" in
which 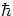 is set equal to 1. See last semester's
lecture on
atomic units.
is set equal to 1. See last semester's
lecture on
atomic units.Lecture 5, Feb 4
Review of quantum mechanics: action, phase,
sum over all possible paths with a phase factor, uncertainty principle,
spin and the Pauli exclusion principle, plane wave solution of
the time-dependent Schrödinger equation,
E = hf = 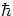 w and
p =
w and
p = 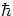 k.
k.
Assigned problem set 2 (engineering of electrostatic precipitator, basic quantum theory).
Problem session 3
Assignment 2 solution: how to compute the gradient in cartesian and spherical coordinates. Using MAPLE: field plots using gradplot, integrals using int, range and properties of variables using assume and about. Given hints for problem set 3.
Lecture 6, Feb 9
- Particle in a box (one dimensional); obtained energy eigenvalues
![]()
where n is a positive integer. Plotted eigenfunctions sin(knx) for n = 1,2,3.
One can write ![]() ,
where
,
where ![]() is the ground state
energy.
is the ground state
energy.
- Harmonic oscillator; eigenvalues
![]()
where n is a positive integer or zero. One can write
![]() where
where
![]() is the ground
state energy, also called the zero-point energy.
is the ground
state energy, also called the zero-point energy.
- Kepler problem (hydrogen atom, neglecting spin and relativistic correction); there are bound states (E<0) that correspond classically to elliptic orbits, and scattering states (E>0) that correspond classically to hyperbolic orbits. The bound state eigenvalues are given by Balmer's formula
![]()
where n is a positive integer and
![]() is the ground state energy.
For hydrogen,
is the ground state energy.
For hydrogen, ![]() eV.
eV.
- Schrödinger equation for a many-electron atom:
![]()
![]() is the potential due to the nucleus:
is the potential due to the nucleus:
![]()
and ![]() is the coulomb potential
of interaction between the electrons:
is the coulomb potential
of interaction between the electrons:
![]()
where ![]() and so on. For the
time-independent equation, replace
and so on. For the
time-independent equation, replace ![]() by
by ![]() .
.
The presence of ![]() makes the problem intractable analytically.
Numerical solutions are obtained by starting with the approximation that
each electron moves in the average potential of the others. In this way
accurate results can be obtained not only for atoms, but also for molecules
and chunks of matter.
makes the problem intractable analytically.
Numerical solutions are obtained by starting with the approximation that
each electron moves in the average potential of the others. In this way
accurate results can be obtained not only for atoms, but also for molecules
and chunks of matter.
References:
- Tipler Chapters 36 and 37, or Serway, chapter 29;
- PQRG, page 93;
- Fowler's notes for Phys 252, especially
"Electron in a box" and the rest of the
"Schrödinger equation" section.
Lecture 7, Feb 11
Assigned problem set 4 (complex numbers, coupled pendulums, two-level quantum system)
Demos: two and three coupled pendulums, array of coupled torsion bars, standing waves and traveling waves.
Reviewed complex numbers and the quantum phase factor exp(-iw t). Demonstrated energy transfer and eigenmodes in coupled pendulums, as a model of two coupled quantum states. The wavefunction of the coupled quantum states is
![]()
and the time evolution is given by

Considered the case when ![]() (identical atoms, for instance). There are two eigenstates: one with
(identical atoms, for instance). There are two eigenstates: one with
![]() (bonding orbital,
energy E+V), and one with
(bonding orbital,
energy E+V), and one with ![]() (antibonding orbital, energy E-V). For N atoms, get a band of N
eigenstates of the whole system from each atomic level (eigenstate of one
atom).
(antibonding orbital, energy E-V). For N atoms, get a band of N
eigenstates of the whole system from each atomic level (eigenstate of one
atom).
References:
Question: In the equations for two coupled quantum states (such as atomic orbitals), what is V and does it have dimensions of energy?
Answer: In this case V denotes a number (not a function of x,y,z as it did in electrostatics) and represents an energy of interaction, suitably weighted over the atomic orbitals. For example, consider two hydrogen atoms that share one electron (hydrogen molecular ion). Then, in gaussian units,
![]()
where ![]() denotes the position of the electron,
denotes the position of the electron,
![]() and
and
![]() those of the nuclei
(just protons in this case);
those of the nuclei
(just protons in this case); ![]() and
and
![]() are the atomic eigenfunctions that
were denoted as
are the atomic eigenfunctions that
were denoted as ![]() and
and
![]() for short.
for short.
Problem session 4
Gone over the solution of problem set 3. Shown how to use Maple to work with complex numbers. Given hints for problem set 4.
Lecture 8, Feb 16
Assigned reading: Melissinos, pages 3-7;
Filling of bands. Metals (good conductors), semiconductors and insulators. Reviewed band structure, valence and conduction band, energy gap. In insulators and semiconductors the conductivity is strongly affected by the concentration of carriers: both electrons added to the conduction band and holes left in the valence band act as carriers (of electrical current). Three mechanisms of creating carriers
Reference: Tipler, Chapter 38, up to page 1291.
Lecture 9, Feb 18
Lecture 10, Feb 23
The conductivity ![]() is by definition the proportionality constant in
the microscopic form of Ohm's law,
is by definition the proportionality constant in
the microscopic form of Ohm's law, ![]() , where J is the current
density and E is the electric field. It is related to the microscopic
properties of the conducting medium by Drude's formula
, where J is the current
density and E is the electric field. It is related to the microscopic
properties of the conducting medium by Drude's formula
![]()
To obtain this formula, consider the effect of an electric field E on a
particle of charge e and mass m* in the presence of a frictional
force: the drift velocity vdrift of the particle satisfies
![]() If there Nc particles per unit volume,
the current density is J=Ncevdrift and Drude's formula
follows by comparing with
If there Nc particles per unit volume,
the current density is J=Ncevdrift and Drude's formula
follows by comparing with ![]() .
.
A more general formula is obtained when there are several groups of
electrons (or holes), each with its m* and ![]() just add the conductivities. The most refined quantum statistical treatments yield
formulas of the same type and in addition relate the relaxation time
just add the conductivities. The most refined quantum statistical treatments yield
formulas of the same type and in addition relate the relaxation time
![]() to the microscopic properties of the medium and to the temperature. A very
useful formula is v
to the microscopic properties of the medium and to the temperature. A very
useful formula is v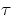 = l, where l is the mean free path and v (notto be confused with
vdrift) is an average speed of the charge carriers in the absence of the field E.
For a classical gas, m*v2=3kBT
gives a good estimate of vat temperature T, but in a metal quantum effects are important and
v is the Fermi velocity, given by (1/2)m*v2 = EF.
(See below for the Fermi energy EF.)
= l, where l is the mean free path and v (notto be confused with
vdrift) is an average speed of the charge carriers in the absence of the field E.
For a classical gas, m*v2=3kBT
gives a good estimate of vat temperature T, but in a metal quantum effects are important and
v is the Fermi velocity, given by (1/2)m*v2 = EF.
(See below for the Fermi energy EF.)
Density of states and occupation probabilities for electrons and holes in solids. Intrinsic semiconductors. The basic formulas are given by Melissinos in eqs. (1.4), (1.5), and (1.7).
![]()
![]()
![]()
Melissinos does not derive the last formula or explain what
![]() is.
See the class notes in the file carriers.pdf
is.
See the class notes in the file carriers.pdf
Assigned reading: Bloomfield, Section 6.3 (incandescent light bulbs).
Lecture 11, Feb 25